| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website https://jnr.elmerpub.com |
Case Report
Volume 15, Number 1, January 2025, pages 51-55
Challenges in Diagnosing and Treating Granulomatous Amebic Encephalitis: A Case Report of Fatal Acanthamoeba spp. Encephalitis in an Immunocompetent Patient
Khaoula Belaidia, b, c, Louhab Nissrina, b, Safae Zahlanea, b, Mohamed Chraaa, b, Najib Kissania, b
aNeurology Department, Mohammed VI University Hospital, Marrakech, Morocco
bMarrakech Medical School, Cadi Ayyad University, Marrakech, Morocco
cCorresponding Author: Khaoula Belaidi, Neurology Department, Mohammed VI University Hospital, Marrakesh, Morocco
Manuscript submitted October 3, 2024, accepted December 20, 2024, published online January 4, 2025
Short title: Challenges in Diagnosing and Treating GAE
doi: https://doi.org/10.14740/jnr854
| Abstract | ▴Top |
Granulomatous amebic encephalitis (GAE) is a rare but fatal infection of the central nervous system with a high mortality rate, due to free-living amoebae that are pathogenic to humans and ubiquitous in the environment. In this paper, we report the case of an immunocompetent adult female with no relevant medical history, who presented with acute symptoms resembling a stroke, including altered mental status, slurred speech, brutal proportional right hemiplegia, facial droop and focal to bilateral tonic-clonic seizures, all concomitant with high fever. Subsequent magnetic resonance imaging of the brain revealed diffuse multiple nodular lesions both above and below the tentorium. The initial cerebrospinal fluid (CSF) profile was of no great significance. However, microscopic examination of CSF was able to identify the presence of amoeboid microorganisms and cyst formation, suggestive of a telluric amoeba’s infection. The patient was then treated with a combination of fluconazole and trimethoprim-sulfamethoxazole, but her neurological state continued to decline until she passed away from GAE. In our case report, we highlight the difficulties clinicians encountered in managing this disease, as the clinical and radiological presentation are nonspecific, in addition to the lack of clear therapeutic guidelines after diagnosis. Our findings point up the urgent need for more precise diagnostic criteria and comprehensive treatment protocols to improve patient outcomes.
Keywords: Granulomatous amebic encephalitis; Central nervous system; Acanthamoeba spp.
| Introduction | ▴Top |
Granulomatous amebic encephalitis (GAE) is a rare central nervous system (CNS) infection that is usually rapidly fatal with a mortality rate exceeding 90% [1]. We have a poor understanding of the disease, and clinical presentation and neuroimaging examination are nonspecific, making it easy to be misdiagnosed. Typically, the onset of symptoms is insidious until overwhelming infection results in rapid severe neurological decline, including seizures, altered levels of consciousness, coma, and death. Diagnosis is challenging due to the nonspecific clinical presentation and radiological features. The optimal treatment for this infection has not been described. Herein, we report a case in which the diagnosis was promptly made by early cerebrospinal fluid (CSF) examination.
| Case Report | ▴Top |
A 48-year-old immunocompetent female with no relevant medical history arrived at the Emergency Department with symptoms resembling a stroke: altered mental status (Glasgow Coma Scale at 12/15), slurred speech, brutal proportional right hemiplegia, facial droop, and focal to bilateral tonic-clonic seizures. Concurrently, our patient presented with a high fever of 40 °C, together with deep deterioration of the general condition and a history of headache and coughing prior to the neurological symptoms. The initial computed tomography (CT) scan did not reveal signs of a stroke. However, subsequent magnetic resonance imaging (MRI) revealed diffuse multiple nodular lesions both above and below the tentorium. The patient was then hospitalized in our Neurology Department for further investigations. After admission, the patient began to demonstrate severe altered levels of consciousness, Glasgow Coma Scale dropped to 9/15, with persistent high fever 39 - 40 °C.
The initial CSF profile was as follows: leukocyte count: 17 cells/mm3 with predominant lymphocytes (80%), erythrocyte count: 778 cells/mm3, glucose concentration: 0.7 g/L, and total protein concentration: 0.4 g/L. Results from Gram staining and culture of CSF were negative. Specific polymerase chain reaction (PCR) testing for herpes simplex virus was also negative, as was the testing with India ink for Cryptococcus neoformans.
Brain MRI scans showed multiple isointense nodular lesions on T1-weighted imaging (Fig. 1), and hyperintense lesions on T2-weighted imaging, with restricted diffusion and without apparent diffusion coefficient (ADC) restriction (Fig. 2). Many of these lesions showed ring enhancement, and some were hemorrhagic; there was meningeal enhancement as well. An infectious origin was initially suspected. A second lumber punction was performed 5 days after; CSF profile was as follows: leukocyte count: 27 cells/mm3 with predominant lymphocytes (80%), erythrocyte count: 0 cells/mm3, glucose concentration: 0.5 g/L, and total protein concentration: 0.6 g/L.
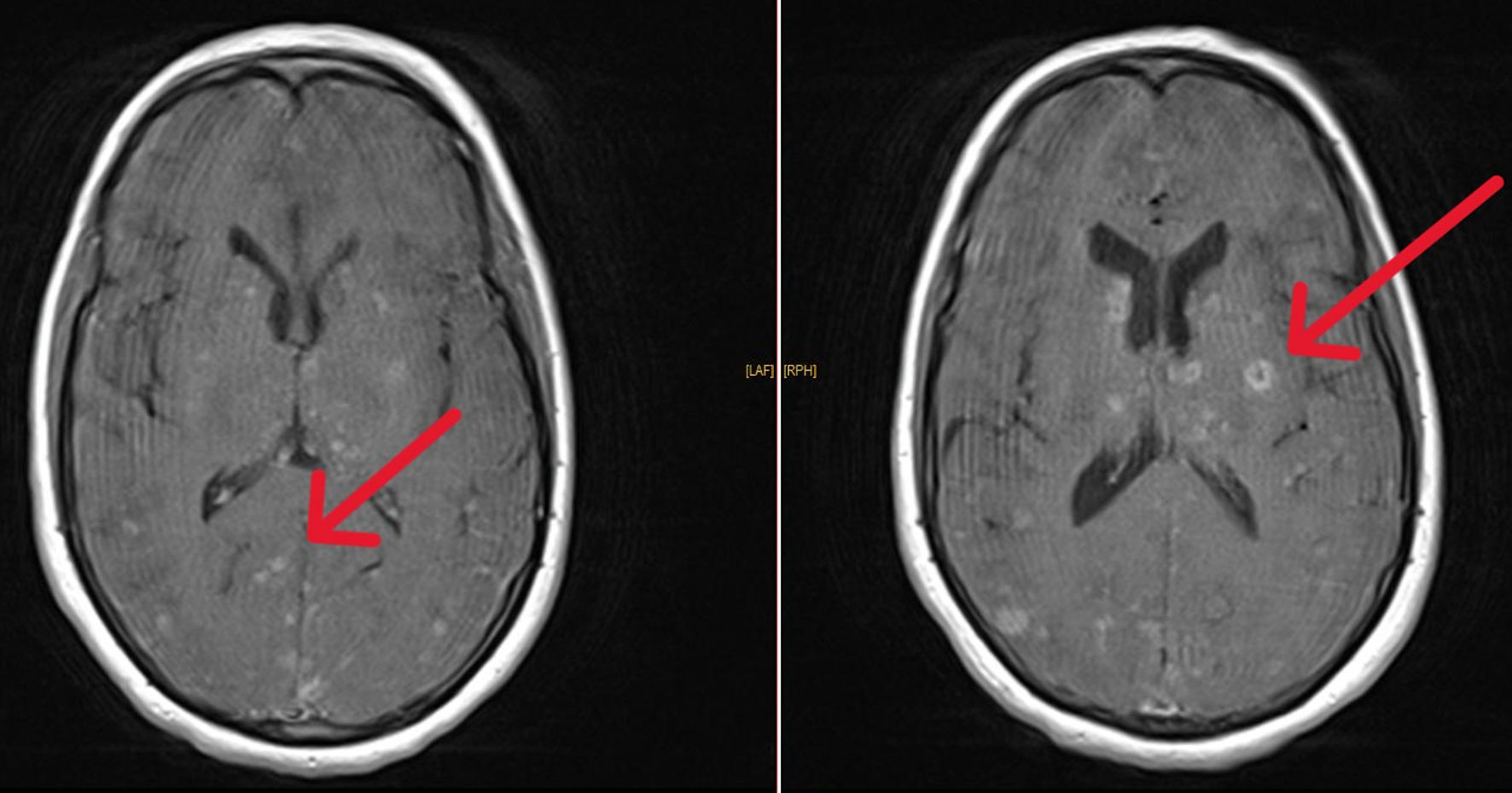 Click for large image | Figure 1. T1-weighted brain MRI sections showing multiple nodular lesions enhancing with gadolinium (arrows). MRI: magnetic resonance imaging. |
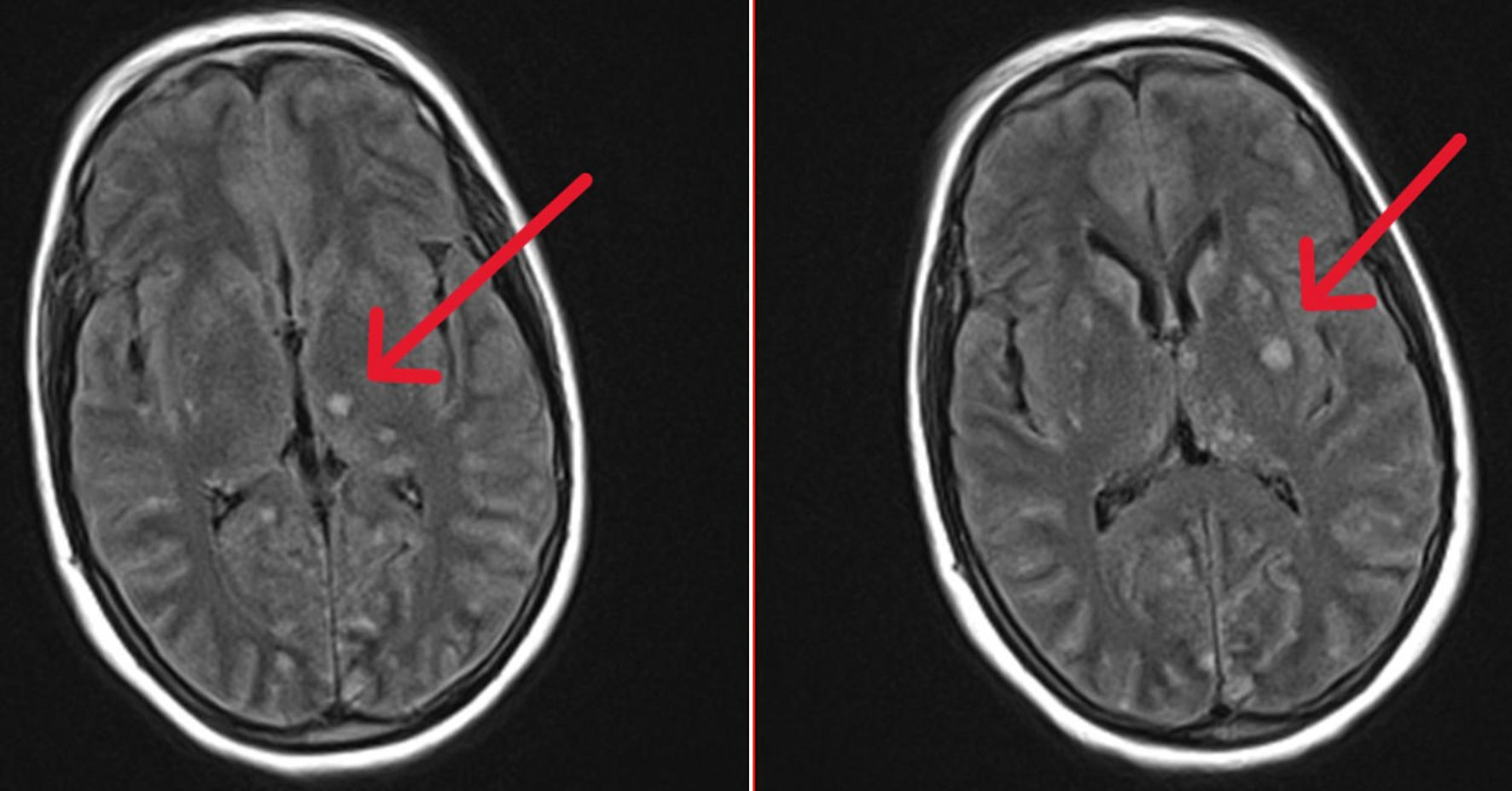 Click for large image | Figure 2. T2-weighted brain MRI sections showing multiple nodular lesions in hypersignal (arrows). MRI: magnetic resonance imaging. |
Microscopic examination of CSF revealed the presence of amoeboid microorganisms and cyst formation, suggestive of a telluric amoeba’s infection (Acanthamoeba sp./Balamuthia mandrillaris) (Fig. 3). To identify the route of entry, and considering the patient’s history of coughing, we performed a CT scan of the lungs. The scan revealed alveolar consolidation with air bronchograms (Fig. 4), highly suggestive of an infectious origin due to the clinical context. The decision was then to start treatment using fluconazole 200 mg twice daily and trimethoprim-sulfamethoxazole 5 mg/kg twice daily.
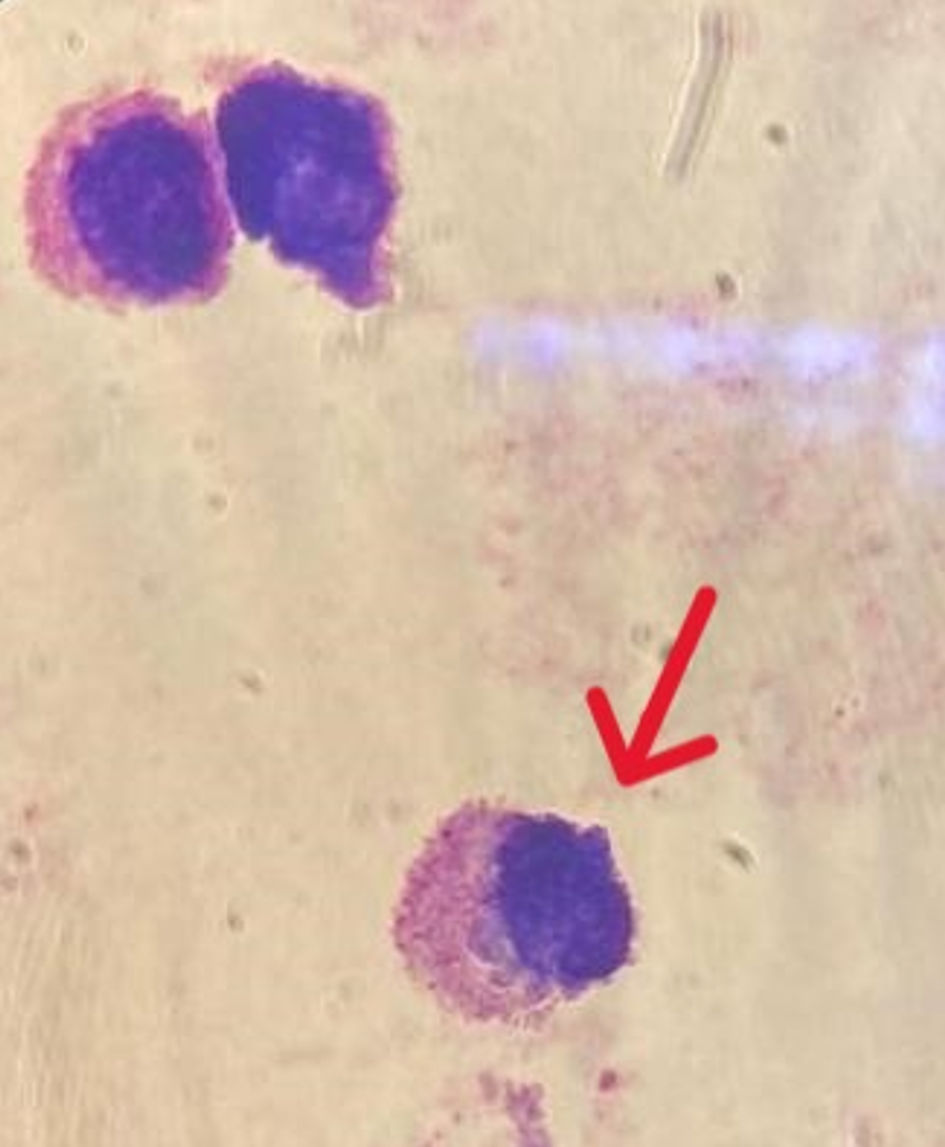 Click for large image | Figure 3. Microscopic examination of CSF after MMG staining showing amoeboid microorganisms (arrow). CSF: cerebrospinal fluid. |
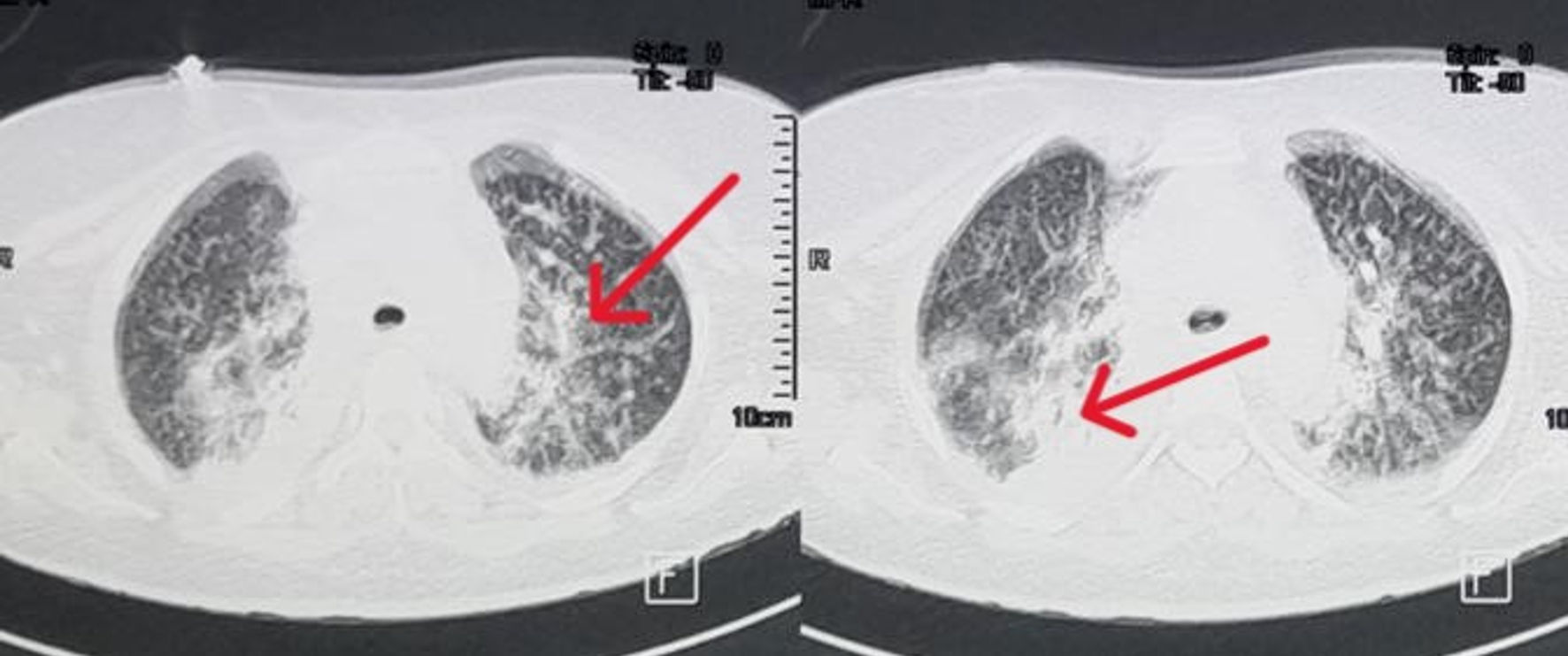 Click for large image | Figure 4. Lung CT scan showing alveolar consolidation with air bronchograms (arrows). CT: computed tomography. |
The patient was transferred to the intensive care unit, where she remained hospitalized for 15 days before she passed away from GAE, 5 weeks after the onset of neurological symptoms.
| Discussion | ▴Top |
GAE is a rare fatal infection of the central nervous system with a mortality rate exceeding 97-98% [2]. From 1990 to 2020, 75 cases of patients with GAE caused by Acanthamoeba spp. have been published [2]. GAE is caused by free-living amoebae (FLA) that are pathogenic to humans and ubiquitous in the natural environment [3]. Three genera of FLA are known to infect human hosts: Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri [3]. GAE is caused by Acanthamoeba or Balamuthia species. Acanthamoeba spp. is ubiquitous and can be found in soil and water, both indoor and outdoor environments. Balamuthia is more likely to be from soil. Entry sites for infection include skin, as well as upper respiratory and urogenital tracts [4]. It has been noted that all those patients infected with Acanthamoeba spp. developed symptoms of upper respiratory tract infection followed by acute meningitis [5].
In our case the portal route is considered to be the upper respiratory tract, based on the respiratory symptoms that the patient had presented with, and pulmonary lesions on CT scan. The origin of FLA is presumed to be outdoor water, as the patient’s entourage reported her direct consumption of raw water from a river near her household. The amoebae break into the nasal mucosa and can penetrate the olfactory tracts, then invade the cribriform plate into the subarachnoid space [4].
The amoebae infiltrate the brain and form granulomatous lesions. The progression of the disease is subacute to chronic, often causing death. Clinical features are typically severe but common and nonspecific. Symptoms include headache, fever, altered mental status, seizures and focal motor deficit [6]. In our case, the patient presented with symptoms resembling a stroke. Consequently, GAE could easily be misdiagnosed as stroke, brain tumors, and other CNS infections, which delays diagnosis and worsens prognosis [7]. At the time of writing, most of the reported cases of GAE occurred in immunocompromised patients [6]. These cases were identified in individuals who had undergone kidney, liver, or allogeneic hematopoietic stem cell transplants, patients receiving immunosuppressive treatments, those with autoimmune disorders, or patients who had tested positive for human immunodeficiency virus (HIV) [8]. On that account, GAE was primarily recognized as an opportunistic infection in immunocompromised hosts; however, several cases have also been reported in healthy patients [2, 6].
In most cases, diagnosing GAE is challenging and often only confirmed postmortem [3]. Definitive diagnosis relies on real-time PCR, immunohistochemistry, and indirect immunofluorescence assays of brain biopsies [9]. CSF examination is crucial for diagnosis. Analyzing CSF can reveal an increased white blood cell count, elevated protein levels, and occasionally the presence of amebas [9-11], as found in our tests. However, the cysts of Balamuthia mandrillaris and Acanthamoeba spp. are morphologically very similar. Generally, they cannot be reliably distinguished without molecular confirmation. Nonetheless, since Acanthamoeba spp. is known to be ubiquitous in raw water and that it causes pneumonia right before the onset of meningoencephalitis [5], we believe that our patient was infected with this particular species. Real-time PCR of CSF may confirm the diagnosis of GAE in the early stage, but amoebae are rarely found [9, 10, 12], and this test is not readily available. Serology for amoeba-specific antibodies is available, but these assays are not widely available, and their specificity for active disease is low since most people have been exposed to this organism at least once in their lifetime [6]. Early diagnosis is crucial for a good prognosis. However, the rarity of the disease and the limited number of reported cases often lead to delayed diagnosis or even misdiagnosis of GAE.
There is no standard therapeutic recommendation for GAE. In most surviving cases various drug combinations were used. Since 2000, 23 surviving cases of Acanthamoeba encephalitis have been reported [2, 5, 6, 9, 12-16]. Fourteen of these cases occurred in healthy, immunocompetent patients [2, 6, 13-15]. Two involved post-transplantation patients (hematopoietic stem cell transplant [9] and liver transplant [8]). Two patients were HIV-positive [2, 12], and the remaining five cases involved individuals with different underlying conditions: disseminated tuberculosis [2], seizure disorder [6], ulcerative colitis [9], chronic malnutrition [2] and acute lymphoblastic leukemia [17]. In each of these cases, a different combination of medications was used. Trimethoprim-sulfamethoxazole, rifampicin, and ketoconazole or fluconazole were the most common agents administered, often simultaneously in surviving cases [2, 6]. Other medications, such as pentamidine, sulfadiazine, pyrimethamine, voriconazole, azithromycin, miltefosine, and metronidazole, were also used but not as frequently [2, 6]. The same drugs were used in cases where patients did not survive, making it difficult to draw reliable conclusions about the efficacy of any particular drug combination. However, trimethoprim-sulfamethoxazole has the highest frequency of drug usage, fluconazole is the most commonly used azole in GAE treatment, and ketoconazole is proved to be the most effective azole [18]. Miltefosine is an anti-parasitic agent recommended by the United States Centers for Disease Control and Prevention for treatment of infections secondary to Acanthamoeba spp., along with pentamidine, flucytosine, fluconazole, and sulfadiazine [1]. It has shown moderate in vitro efficacy against Acanthamoeba spp. [1]. Additionally, introducing miltefosine in drug combinations for the treatment of Acanthamoeba spp. GAE is thought to have improved survival [19]. However, the success of miltefosine treatment remains limited to a small number of reported cases. In our case, we attempted to cure the patient using a combination of trimethoprim-sulfamethoxazole and fluconazole in high doses. The patient remained comatose for 20 days after diagnosis before she succumbed to death. Based on the data gathered from this case report, it remains difficult to establish a definitive drug combination to enhance survival rates among patients with GAE. However, drugs like fluconazole, trimethoprim-sulfamethoxazole and miltefosine have shown promise as an effective therapeutic regimen, offering better chances of survival. We recommend that future studies explore the potential benefit of multi-drug regimens, including those drugs. These combinations have been used in reported cases with survival, but their efficacy requires further investigation through well-designed clinical studies.
Conclusions
Further research and clinical studies are needed to improve diagnostic methods and establish effective treatment strategies for GAE. Cases like ours can provide valuable information to the medical community and may help raise awareness of this uncommon but severe disease. Simple microscopic examination of CSF can sometimes help identify the presence of amoeboid microorganisms. Early diagnosis and prolonged multi-agent treatment improve prognosis and increase chances of survival.
Acknowledgments
We would like to express our gratitude to the staff of the Neurology Department at Mohammed VI University Hospital in Marrakesh for their support and the care provided to the deceased. We extend our thanks to the teams in the Intensive Care Unit and the Departments of Microbiology and Parasitology, for their invaluable assistance in the diagnosis and management of this case. Finally, we are deeply appreciative of the patient’s family for granting us permission to publish this case.
Financial Disclosure
No funding sources or financial support received for the paper.
Conflict of Interest
No potential conflict of interest related to the paper or the authors.
Informed Consent
Informed consent was obtained from the patient’s family.
Author Contributions
Conception and design: Khaoula Belaidi, Najib Kissani, Louhab Nissrin, Mohamed Chraa, Safae Zahlane. Supervision: Najib Kissani, Louhab Nissrin, Mohamed Chraa, Safae Zahlane. Case study: Khaoula Belaidi. Literature review: Khaoula Belaidi. Writing: Khaoula Belaidi. Critical review: Najib Kissani, Louhab Nissrin, Mohamed Chraa, Safae Zahlane.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
CNS: central nervous system; CSF: cerebrospinal fluid; CT: computed tomography; FLA: free-living amoebae; HIV: human immunodeficiency virus; GAE: granulomatous amebic encephalitis; MRI: magnetic resonance imaging; PCR: polymerase chain reaction
| References | ▴Top |
- Spottiswoode N, Haston JC, Hanners NW, Gruenberg K, Kim A, DeRisi JL, Wilson MR. Challenges and advances in the medical treatment of granulomatous amebic encephalitis. Ther Adv Infect Dis. 2024;11:20499361241228340.
doi pubmed - Kot K, Lanocha-Arendarczyk N, Kosik-Bogacka D. Immunopathogenicity of acanthamoeba spp. in the brain and lungs. Int J Mol Sci. 2021;22(3):1261.
doi pubmed - Matsui T, Maeda T, Kusakabe S, Arita H, Yagita K, Morii E, Kanakura Y. A case report of granulomatous amoebic encephalitis by Group 1 Acanthamoeba genotype T18 diagnosed by the combination of morphological examination and genetic analysis. Diagn Pathol. 2018;13(1):27.
doi pubmed - McKellar MS, Mehta LR, Greenlee JE, Hale DC, Booton GC, Kelly DJ, Fuerst PA, et al. Fatal granulomatous Acanthamoeba encephalitis mimicking a stroke, diagnosed by correlation of results of sequential magnetic resonance imaging, biopsy, in vitro culture, immunofluorescence analysis, and molecular analysis. J Clin Microbiol. 2006;44(11):4265-4269.
doi pubmed - Chan A, Smith S, Tan E, Kuruvath S. Case report: first successful treatment of acanthamoeba brain abscess with combination surgical excision and miltefosine-led antimicrobial therapy. Am J Trop Med Hyg. 2022;106(3):861-866.
doi pubmed - Zamora A, Henderson H, Swiatlo E. Acanthamoeba encephalitis: A Case Report and Review of Therapy. Surg Neurol Int. 2014;5:68.
doi pubmed - Lee DC, Fiester SE, Madeline LA, Fulcher JW, Ward ME, Schammel CM, Hakimi RK. Acanthamoeba spp. and Balamuthia mandrillaris leading to fatal granulomatous amebic encephalitis. Forensic Sci Med Pathol. 2020;16(1):171-176.
doi pubmed - Fung KT, Dhillon AP, McLaughlin JE, Lucas SB, Davidson B, Rolles K, Patch D, et al. Cure of Acanthamoeba cerebral abscess in a liver transplant patient. Liver Transpl. 2008;14(3):308-312.
doi pubmed - Keane NA, Lane LM, Canniff E, Hare D, Doran S, Wallace E, Hutchinson S, et al. A surviving case of acanthamoeba granulomatous amebic encephalitis in a hematopoietic stem cell transplant recipient. Am J Case Rep. 2020;21:e923219.
doi pubmed - Salameh A, Bello N, Becker J, Zangeneh T. Fatal granulomatous amoebic encephalitis caused by acanthamoeba in a patient with kidney transplant: a case report. Open Forum Infect Dis. 2015;2(3):ofv104.
doi pubmed - Walochnik J, Aichelburg A, Assadian O, Steuer A, Visvesvara G, Vetter N, Aspock H. Granulomatous amoebic encephalitis caused by Acanthamoeba amoebae of genotype T2 in a human immunodeficiency virus-negative patient. J Clin Microbiol. 2008;46(1):338-340.
doi pubmed - El Sahly H, Udayamurthy M, Parkerson G, Hasbun R. Survival of an AIDS patient after infection with Acanthamoeba sp. of the central nervous system. Infection. 2017;45(5):715-718.
doi pubmed - Modica S, Miracco C, Cusi MG, Tordini G, Muzii VF, Iacoangeli F, Nocentini C, et al. Non-granulomatous cerebellar infection by Acanthamoeba spp. in an immunocompetent host. Infection. 2018;46(6):885-889.
doi pubmed - Webster D, Umar I, Kolyvas G, Bilbao J, Guiot MC, Duplisea K, Qvarnstrom Y, et al. Treatment of granulomatous amoebic encephalitis with voriconazole and miltefosine in an immunocompetent soldier. Am J Trop Med Hyg. 2012;87(4):715-718.
doi pubmed - Lackner P, Beer R, Broessner G, Helbok R, Pfausler B, Brenneis C, Auer H, et al. Acute granulomatous acanthamoeba encephalitis in an immunocompetent patient. Neurocrit Care. 2010;12(1):91-94.
doi pubmed - Singhal T, Bajpai A, Kalra V, Kabra SK, Samantaray JC, Satpathy G, Gupta AK. Successful treatment of Acanthamoeba meningitis with combination oral antimicrobials. Pediatr Infect Dis J. 2001;20(6):623-627.
doi pubmed - Maritschnegg P, Sovinz P, Lackner H, Benesch M, Nebl A, Schwinger W, Walochnik J, et al. Granulomatous amebic encephalitis in a child with acute lymphoblastic leukemia successfully treated with multimodal antimicrobial therapy and hyperbaric oxygen. J Clin Microbiol. 2011;49(1):446-448.
doi pubmed - Taravaud A, Fechtali-Moute Z, Loiseau PM, Pomel S. Drugs used for the treatment of cerebral and disseminated infections caused by free-living amoebae. Clin Transl Sci. 2021;14(3):791-805.
doi pubmed - Aichelburg AC, Walochnik J, Assadian O, Prosch H, Steuer A, Perneczky G, Visvesvara GS, et al. Successful treatment of disseminated Acanthamoeba sp. infection with miltefosine. Emerg Infect Dis. 2008;14(11):1743-1746.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.