| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website https://jnr.elmerpub.com |
Case Report
Volume 15, Number 1, January 2025, pages 47-50
Spontaneous Subdural Hematoma Secondary to Influenza Vaccine-Induced Immune Thrombocytopenia
Hesham Kelania, j, Izabella Beniaminovab, i, Emina Dzaficc, i, Priya Srivastavad, Noor El-Deen Farghalyd, Saranjeet Kaurc, Lauren Hatchere, Mohammad Jadidia, Marie Mikhailf, Diana Greene-Chandosg, Lisa R. Merlina, h
aDepartment of Neurology, SUNY Downstate Health Sciences University at One Brooklyn Health, Brooklyn, NY, USA
bNYIT College of Osteopathic Medicine, Old Westbury, NY, USA
cTouro College of Osteopathic Medicine, Harlem, NY, USA
dSaba University School of Medicine, The Bottom, Saba
eDepartment of Neurology, University of New Mexico, Albuquerque, NM, USA
fDepartment of Medicine, New York Presbyterian Brooklyn Methodist, Brooklyn, NY, USA
gDepartment of Neurology, School of Medicine, University of Saint Louis, Saint Louis, MO, USA
hDepartment of Neurology, Physiology, and Pharmacology, SUNY Downstate Health Sciences University, Brooklyn, NY, USA
iThese authors contributed equally to this study.
jCorresponding Author: Hesham Kelani, Department of Neurology, SUNY Downstate University at One Brooklyn Health, Brooklyn, NY 11212, USA
Manuscript submitted September 4, 2024, accepted December 30, 2024, published online January 8, 2025
Short title: Spontaneous SDH Following Flu Vaccine
doi: https://doi.org/10.14740/jnr853
| Abstract | ▴Top |
Influenza vaccine-induced immune thrombocytopenia (ITP), though rare, can present with a wide spectrum of clinical manifestations, ranging from mild asymptomatic thrombocytopenia to life-threatening complications, such as gastrointestinal (GI) bleeding, diffuse alveolar hemorrhage (DAH), or intracranial hemorrhage (ICH), particularly when platelet count drops below a critical level of less than 5,000/µL. We report a rare case of spontaneous subdural hematoma (SDH) due to severe ITP following the influenza vaccine that showed a dramatic response to high-dose methylprednisolone and intravenous immunoglobulin (IVIG), with platelet count returning to normal and complete resolution of symptoms by the third day of therapy initiation.
Keywords: Subdural hematoma; Influenza vaccine; Immune thrombocytopenia; Methylprednisolone; IVIG
| Introduction | ▴Top |
Influenza vaccination has been reported in previous studies to cause vaccination-induced adverse reactions, including Guillain-Barre syndrome, convulsions, Bell’s palsy, and thrombocytopenia [1-3]. On the other hand, immune thrombocytopenia (ITP) has been associated with vaccinations such as measles-mumps-rubella (MMR), hepatitis A, hepatitis B, diphtheria-tetanus-acellular pertussis (DTaP), and varicella [4]. Although ITP induced by influenza vaccination is uncommon, eight cases of influenza vaccine-induced thrombocytopenia have been described in the French PharmacoVigilance database as well as three in Seichokai Fuchu Hospital, Japan. Due to its rarity, the pathogenesis of vaccine-related thrombocytopenia is unclear with no apparent risk factors, but some cases have been linked to secondary ITP [3, 5-7].
Mild thrombocytopenia is almost always asymptomatic, while severe thrombocytopenia can cause varying degrees of bleeding, such as skin petechiae, hematomas, and bloody stool. Persistent severe thrombocytopenia can lead to very low platelet counts resulting in potentially fatal complications, including subarachnoid hemorrhage, intracerebral bleeding, or diffuse alveolar hemorrhage (DAH) [7, 8]. It has been observed that the onset of these symptoms ranges from mild to severe and follows a strict time-dependent relationship, developing within 4 - 35 days after vaccination [7, 9]. While patients with primary ITP often require long-term therapy due to the chronic progression of their symptoms [10], patients with vaccine-induced ITP usually respond dramatically to glucocorticoids within a few days [9, 11, 12].
| Case Report | ▴Top |
Investigations
A 70-year-old male presented to the hospital with left-sided weakness, numbness, and generalized, scattered bruising with no history of trauma. His past medical history included hypertension, hyperlipidemia, coronary artery disease, type II diabetes mellitus (DM), chronic kidney disease (CKD), and atrial fibrillation. He was not on direct anticoagulation; however, he was taking 81 mg of aspirin daily. Four weeks prior to admission, the patient was in his usual state of health and received an influenza vaccine. One to two days later, he noticed bruising and swelling around the immunization site on his right upper extremity. Over the subsequent 2 weeks, he developed scattered bruising across his body. On the advice of his primary care physician, he discontinued aspirin, but his symptoms persisted.
Seven to ten days prior to admission, he developed sensory and motor deficits, including dysphagia, sialorrhea, and a progressively worsening headache accompanied by extremity weakness. Upon admission, a non-contrast computed tomography (CT) of the head was obtained, revealing an acute right-sided subdural hematoma (SDH) with mild parafalcine herniation and brain compression (Figs. 1 and 2). Surgical intervention was not indicated at this time.
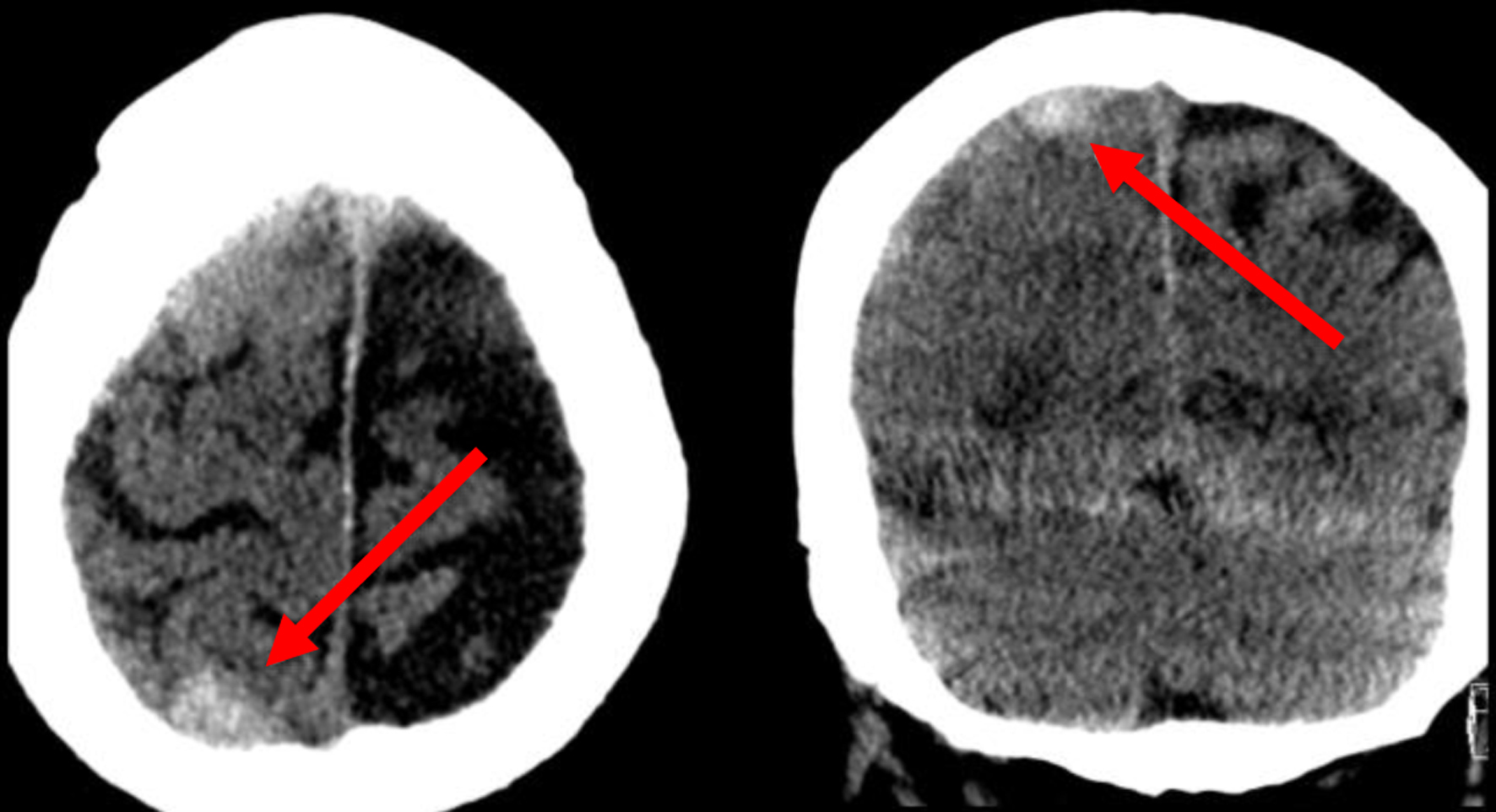 Click for large image | Figure 1. Non-contrast head CT in the axial and coronal planes revealing a right posterior convexity subdural hemorrhage (arrows). CT: computed tomography. |
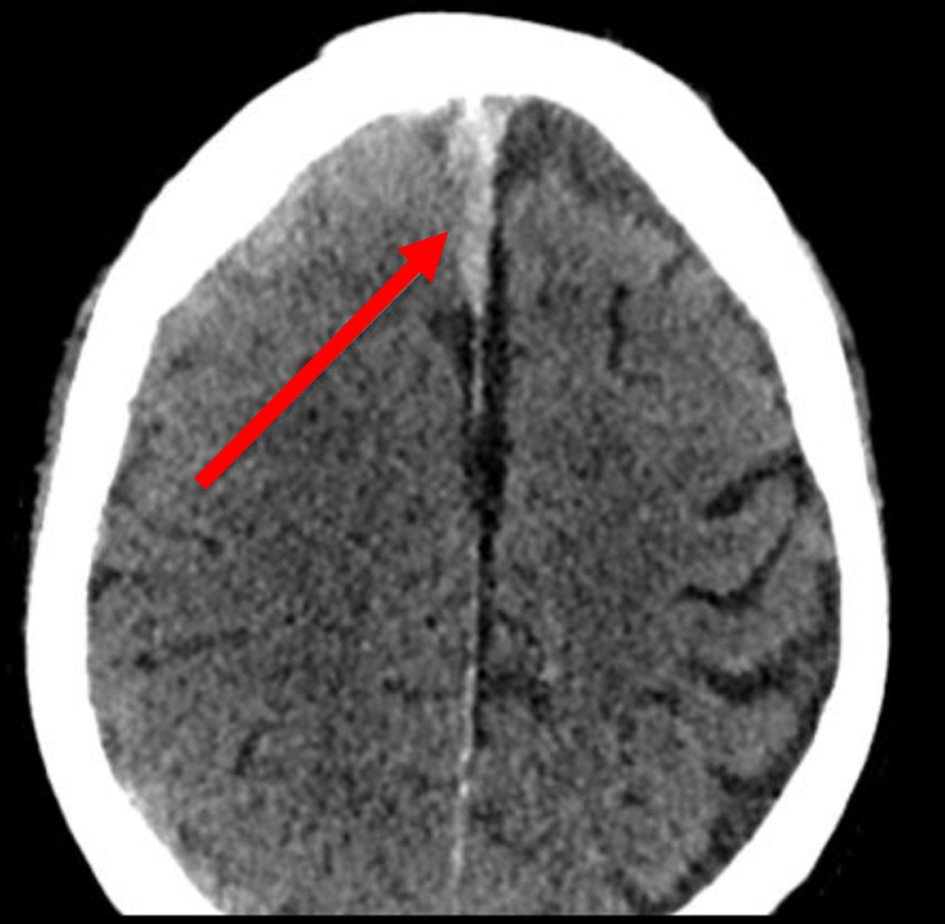 Click for large image | Figure 2. Non-contrast axial CT showing right parafalcine subdural hemorrhage (arrow). CT: computed tomography. |
Diagnosis
Laboratory tests revealed a platelet count of 5,000/µL, which improved only slightly to 18,000/µL after transfusion of two units of platelets before it dropped back to 5,000/µL on repeating laboratory tests 12 h later. This necessitated transfusion of another two units of platelets with improvement of platelet count to 22,000/µL before it dropped again to 9,000/µL on repeated laboratory tests 12 h later. A review of previous laboratory results from months prior showed a normal platelet profile, and the patient’s history was negative for alcohol use, liver disease, or splenomegaly, which was confirmed by an abdominal ultrasound.
Treatment
Suspecting ITP, intravenous high-dose methylprednisolone (30 mg/kg/day for 3 days) was delivered. Over the next 3 days, repeat laboratory tests showed a progressive increase in platelet counts to 35,000, 98,000, and 198,000/µL, respectively. A non-contrast CT of the head showed stable SDH, and the patient’s neuro-examination significantly improved. The influenza vaccine-induced ITP diagnosis was confirmed. The patient was discharged home on oral methylprednisolone (20 mg/kg/day for 4 days).
Follow-up and outcomes
On subsequent outpatient follow-up visit 2 weeks later, platelet count was 227,000/µL and the patient’s neuro-examination returned back to his normal baseline.
| Discussion | ▴Top |
Although not a very common adverse reaction to influenza vaccine, ITP has been recorded with varying degrees of thrombocytopenia ranging from mildly asymptomatic to severe with serious fatal bleeding when platelet count drops to < 5,000/µL. Here, we present a case of spontaneous SDH in a 70-year-old male secondary to severe ITP 4 weeks after receiving an influenza vaccine. This case is unique because of the patient’s delayed neurological presentation, their baseline CKD that may be a confounder for low platelet count and dysfunction, the patient’s age, and lastly, a negative history of adverse reactions from influenza vaccines in the past.
The progressive course of the disease started as localized bruising and swelling around the injection site 24 h after injection, followed by diffuse skin rash and ecchymosis over the following 2 weeks. Subsequently, the onset of neurological symptoms and spontaneous SDH occurred after 4 weeks. This correlates with the temporal drop in the platelet count over this period, with the lowest count of < 5,000/µL at admission. Immediate correction with two units of platelets showed an insignificant rise in the platelet count from < 5,000 to < 18,000/µL before it dropped back to 5,000/µL on repeating laboratory tests 12 h later. Another transfusion of two units of platelets was only able to improve platelet count to 22,000/µL before it dropped again to 9,000/µL on repeated laboratory tests 12 h later. Abdominal ultrasound showed the absence of splenomegaly; this ruled out a primary ITP process. Following treatment with intravenous high-dose methylprednisolone, the platelet count increased dramatically. These clinical events together confirmed the diagnosis of influenza vaccine-induced ITP.
While this case of vaccine-induced ITP resolved by day 3 of intravenous high-dose methylprednisolone initiation, additional therapy is warranted when symptoms are severe or persistent. Current standard of care for primary and secondary ITP includes high-dose corticosteroids with intravenous immunoglobulin (IVIG) therapy, although platelet transfusions are required in instances with active bleeding, and plasmapheresis may be indicated in fulminant cases [13, 14]. While rare, several instances of severe manifestations of ITP following influenza vaccinations have been reported, with symptoms ranging from gross hematuria to DAH leading to acute respiratory failure. In such cases, patients have been shown to respond dramatically to a combination of high-dose steroids, IVIG, and platelet transfusion, with complete resolution of symptoms and normalized platelet counts without necessitation of invasive interventions [8, 12, 13]. However, both primary and post-vaccine ITP processes can become chronic, requiring extended therapy and possible splenectomy [15]. While antineoplastics such as rituximab have been used in intractable cases, studies have demonstrated limited efficacy with slow onset of action and notable adverse side effects [16].
Our case highlights the potentially catastrophic outcomes that can come from undiagnosed ITP, and underscores the critical need for early detection and treatment. Early monitoring of platelet counts post-vaccination may be warranted in individuals with a prior history of autoimmune diseases or previous episodes of thrombocytopenia. Establishing protocols for early intervention upon the first signs of thrombocytopenia, as well as patient education to recognize the symptoms of severe thrombocytopenia following vaccination, can enhance early detection and prompt medical attention before significant complications arise.
Learning points
Influenza vaccine-induced ITP should be suspected once a patient presents with progressive symptoms such as diffuse erythema, swelling, and ecchymoses in the setting of low platelet count non-responsive to platelet transfusion following influenza vaccine. Early treatment with intravenous high-dose methylprednisolone is required to prevent the development of delayed fatal complications when platelet count drops below a critical level. This treatment is also sufficient to resolve the condition without the need for additional therapy.
Acknowledgments
The authors express deep gratitude to the patient for allowing us the opportunity to be involved in their care.
Financial Disclosure
None of the authors involved in this article, nor their immediate family members, have received financial compensation from or hold stock options in any commercial entity related directly or indirectly to the topic of this article. Additionally, no financial support or assistance was provided to the authors for their contribution to this work.
Conflict of Interest
The authors declare that they have no conflict of interest to disclose.
Informed Consent
Informed consent was obtained from this patient.
Author Contributions
Hesham Kelani: conceptualization, study design, drafting, and revisions. Izabella Beniaminova: data collection, critical analysis and drafting. Emina Dzafic: critical analysis, drafting, and manuscript revision. Priya Srivastava: data collection and critical analysis. Noor El-Deen Farghaly: data collection and critical analysis. Saranjeet Kaur: data collection and critical analysis. Lauren Hatcher: data collection, critical analysis, and drafting. Mohammad Jadidi: data collection, critical analysis, and drafting. Marie Mikhail: data collection and critical analysis. Diana Greene-Chandos: supervision, critical revisions, and final approval. Lisa R. Merlina: supervision, critical revisions, and final approval.
Data Availability
The data for this report were obtained through the Brookdale Institutional Review Board (IRB). The IRB committee classified this study as exempt from approval. The data are not publicly available, but further details and contributions for access can be obtained by contacting the corresponding author.
Abbreviations
CKD: chronic kidney disease; DAH: diffuse alveolar hemorrhage; DTaP: diphtheria-tetanus-acellular pertussis; GI: gastrointestinal; ICH: intracranial hemorrhage; ITP: immune thrombocytopenia; IVIG: intravenous immunoglobulin; MMR: measles-mumps-rubella; SDH: subdural hematoma
| References | ▴Top |
- Dieleman J, Romio S, Johansen K, Weibel D, Bonhoeffer J, Sturkenboom M, VAESCO-GBS Case-Control Study Group. Guillain-Barre syndrome and adjuvanted pandemic influenza A (H1N1) 2009 vaccine: multinational case-control study in Europe. BMJ. 2011;343:d3908.
doi pubmed - Martin Arias LH, Sanz R, Sainz M, Treceno C, Carvajal A. Guillain-Barre syndrome and influenza vaccines: A meta-analysis. Vaccine. 2015;33(31):3773-3778.
doi pubmed - Huang WT, Huang WI, Huang YW, Hsu CW, Chuang JH. The reporting completeness of a passive safety surveillance system for pandemic (H1N1) 2009 vaccines: a capture-recapture analysis. Vaccine. 2012;30(12):2168-2172.
doi pubmed - Rinaldi M, Perricone C, Ortega-Hernandez OD, Perricone R, Shoenfeld Y. Immune thrombocytopaenic purpura: an autoimmune cross-link between infections and vaccines. Lupus. 2014;23(6):554-567.
doi pubmed - Moulis G, Sommet A, Sailler L, Lapeyre-Mestre M, Montastruc JL, French Association Of Regional Pharmacovigilance Centers. Drug-induced immune thrombocytopenia: a descriptive survey in the French PharmacoVigilance database. Platelets. 2012;23(6):490-494.
doi pubmed - Klok FA, Pai M, Huisman MV, Makris M. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2022;9(1):e73-e80.
doi pubmed - Perricone C, Ceccarelli F, Nesher G, Borella E, Odeh Q, Conti F, Shoenfeld Y, et al. Immune thrombocytopenic purpura (ITP) associated with vaccinations: a review of reported cases. Immunol Res. 2014;60(2-3):226-235.
doi pubmed - Yamamoto Y, Ohara Y, Iwai A, Hara R, Matsuki T, Fukushima K, Oshitani Y, et al. Influenza vaccination-associated acute thrombocytopenia and diffuse alveolar hemorrhage. Intern Med. 2020;59(13):1633-1637.
doi pubmed - Nagasaki J, Manabe M, Ido K, Ichihara H, Aoyama Y, Ohta T, Furukawa Y, et al. Postinfluenza vaccination idiopathic thrombocytopenic purpura in three elderly patients. Case Rep Hematol. 2016;2016:7913092.
doi pubmed - Nazi I, Kelton JG, Larche M, Snider DP, Heddle NM, Crowther MA, Cook RJ, et al. The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood. 2013;122(11):1946-1953.
doi pubmed - Ikegame K, Kaida K, Fujioka T, Kawakami M, Hasei H, Inoue T, Taniguchi Y, et al. Idiopathic thrombocytopenic purpura after influenza vaccination in a bone marrow transplantation recipient. Bone Marrow Transplant. 2006;38(4):323-324.
doi pubmed - Satoh E, Nei T, Kuzu S, Chubachi K, Nojima D, Taniuchi N, Yamano Y, et al. Acute lung injury accompanying alveolar hemorrhage associated with flu vaccination in the elderly. Intern Med. 2015;54(24):3193-3196.
doi pubmed - Almohammadi A, Lundin MS, Abro C, Hrinczenko B. Epistaxis and gross haematuria with severe thrombocytopaenia associated with influenza vaccination. BMJ Case Rep. 2019;12(5):e229423.
doi pubmed - Provan D, Newland A. Fifty years of idiopathic thrombocytopenic purpura (ITP): management of refractory itp in adults. Br J Haematol. 2002;118(4):933-944.
doi pubmed - Weiner M, Rodriguez-Vigouroux R, Masouridi-Levrat S, Samii K. Very severe immune thrombocytopenia following SARS-CoV-2 vaccination requiring splenectomy: a case report. Thromb J. 2022;20(1):45.
doi pubmed - Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.