Figures
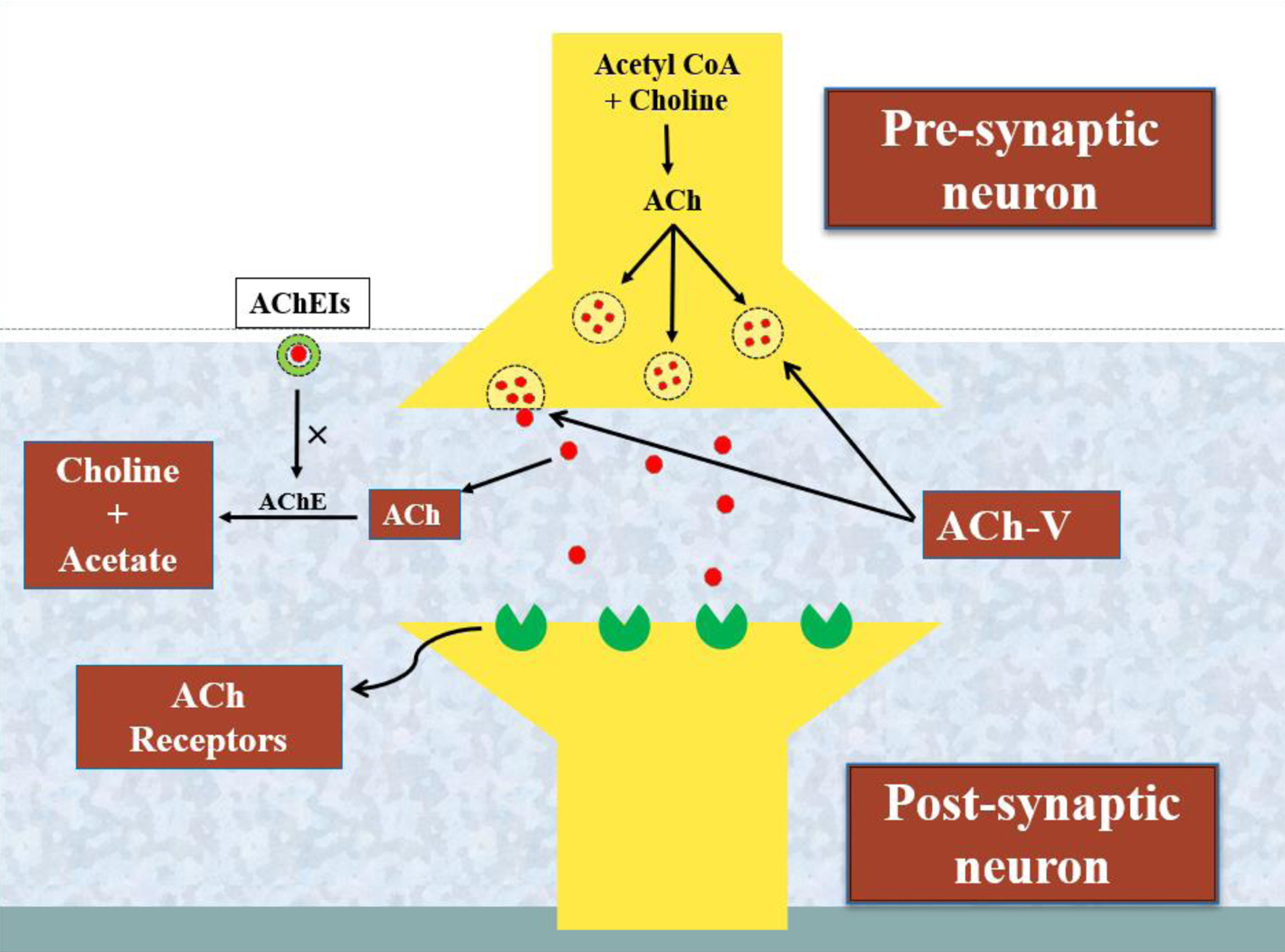
Figure 1. A schematic representation of the activity of acetylcholinesterase and the processing of the neurotransmitter acetylcholine. The enzyme choline acetyltransferase converts acetyl-CoA and choline into acetylcholine (ACh). Furthermore, ACh is released from ACh vesicles (ACh-V) to bind ACh receptors and performs a variety of neurological tasks that are impaired by the degrading action of another enzyme, acetylcholinesterase (AChE). In this way, the acetylcholinesterase inhibitors (AChEIs) reverse the brain functioning by blocking the activity of AChE in Alzheimer’s disease (AD).
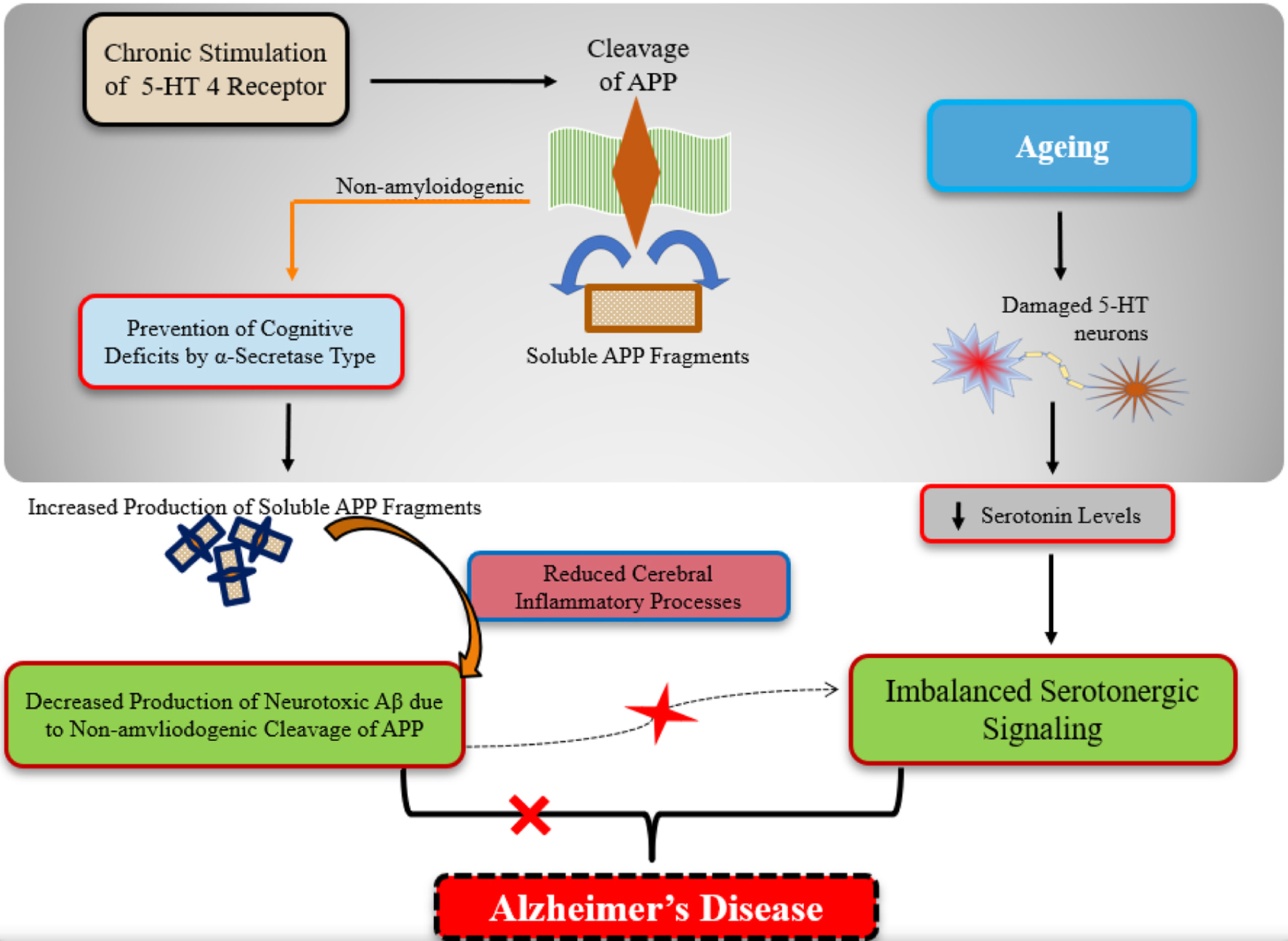
Figure 2. A diagrammatic presentation of the role of the serotonergic system signaling in aging and Alzheimer’s disease (AD). As we grow older, the serotonergic neurons tend to get damaged which leads to impair the normal serotonergic signaling in elderly. In case of AD, the chronic stimulation of the serotonin type-4 receptor leads to the non-amyloidogenic α-secretase cleavage of amyloid precursor proteins (APPs). This cleavage type results in the increased production of non-neurotoxic beta-amyloid (Aβ) fragments (i.e., soluble forms) and the depleted production of neurotoxic Aβ (i.e., non-soluble forms).
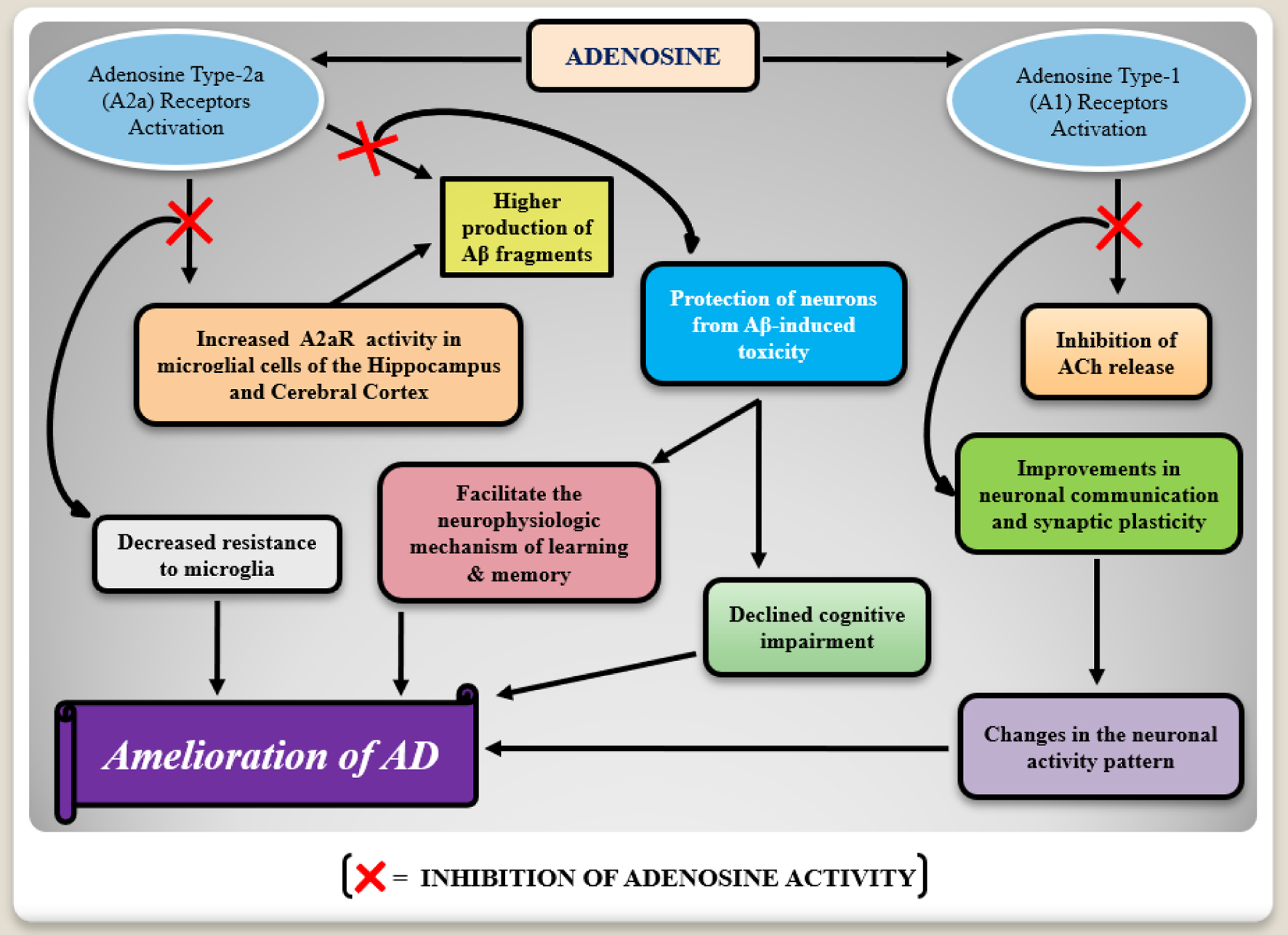
Figure 3. A flow chart mechanism of the adenosine type-1 (A1) and type-2 (A2) receptors involvement in Alzheimer’s disease (AD) pathophysiology. Adenosine receptors activation seems to induce degenerative consequences. Increased expressions of type-2 adenosine receptors (A2aR) in microglia may favor the production of neurotoxic beta-amyloid (Aβ) proteins, whereas type-1 adenosine receptors are responsible for acetylcholine (ACh) release inhibition which leads to the degenerative changes in the neuronal activity pattern in AD. In this way, the inhibition of these receptors activity presents the cognition improvement.
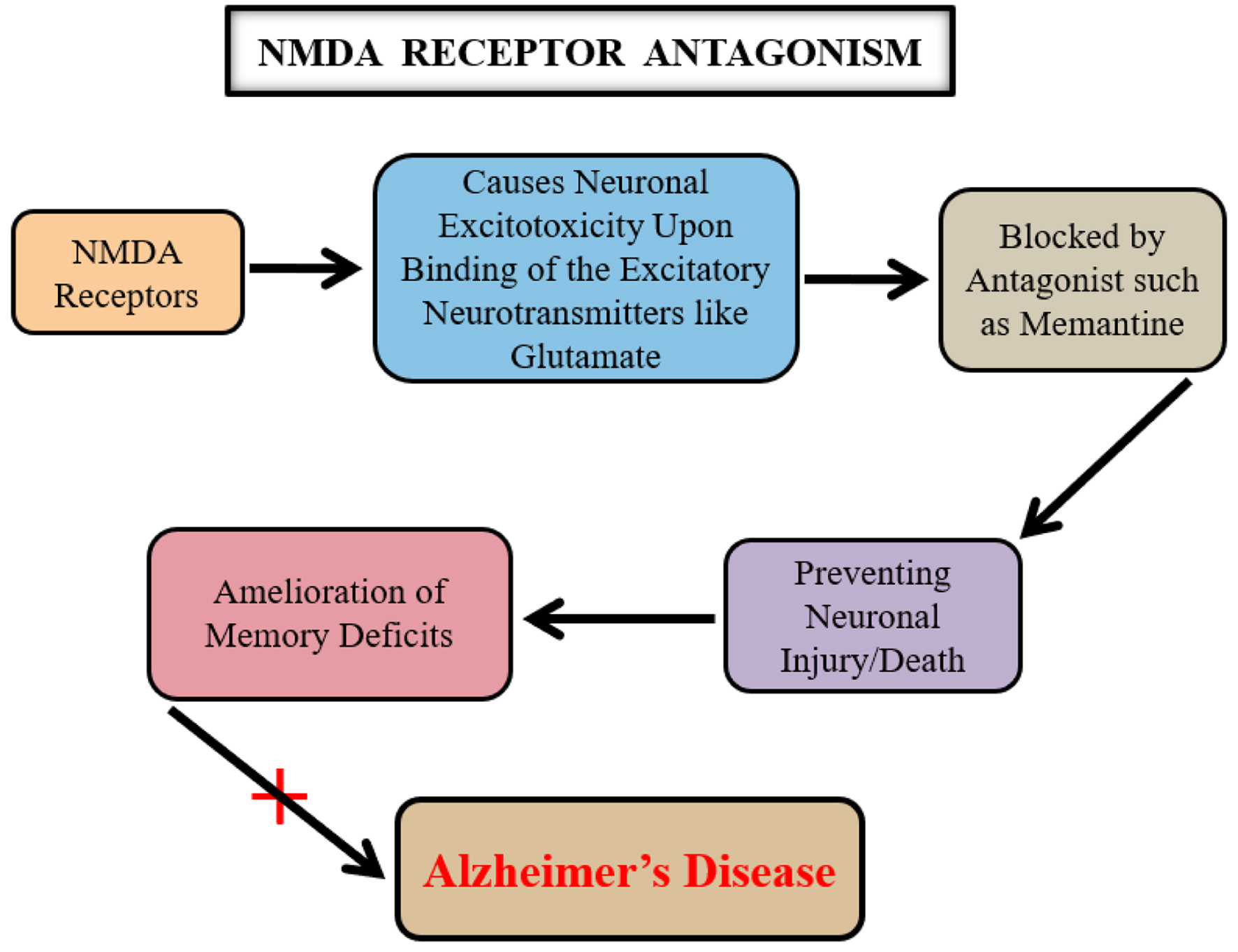
Figure 4. A key relationship among the N-methyl D-aspartate (NMDA) receptor signaling, glutamate, and Alzheimer’s disease (AD). The neurotransmitter glutamate causes neuronal excito-toxicity upon its binding to the NMDA receptors. A prolonged activation of the NMDA glutamate receptors leads to the neuronal dysfunctioning which is potentially inhibited by using an NMDA receptor antagonist such as memantine. This mechanistic effect of memantine is therefore counted as beneficial in AD.
Tables
Table 1. Important Signaling Cascades and Their Primary Targets in the Pathophysiology of AD [9-14]
| Novel signaling pathways | Major signaling components | Effectors | Effects |
|---|
| AD: Alzheimer’s disease; GABA: gamma-amino butyric acid; NMDA: N-methyl D-aspartate. |
| Cholinergic signaling pathway | Acetylcholinesterase, an enzyme | Donepezil | Inhibitory |
| NMDA-glutamate signaling pathway | Glutamate, a neurotransmitter | Memantine | Antagonism |
| Serotonin signaling pathway | Serotonin, a monoamine | Clozapine | Modulatory |
| GABAergic signaling pathway | GABA, a neurotransmitter | Homo-taurine | Upregulatory |
| Adenosine signaling pathway | Adenosine, a nucleoside | Caffeine | Modulatory/antagonism |
| Histamine signaling pathway | Histamine, an immunogenic | Dimebolin | Antihistamine |
Table 2. Drug Entities Under Phase 3 Clinical Trials Against Possible Molecular Cascades for the Potential Futuristic Therapeutics in AD Treatment Scenario
| Molecular signaling cascades | Agents in phase 3 clinical trials | References |
|---|
| AD: Alzheimer’s disease; GABA: gamma-amino butyric acid; NMDA: N-methyl D-aspartate. |
| Cholinergic system signaling | Blarcamesine (ANAVEX2-73) | 18-20 |
| KarXT | 21, 22 |
| NMDA receptor signaling | AXS-05 | 20, 22, 23 |
| Serotonin receptor signaling | Masupirdine | 21, 22 |
| OPC-34712 | 19-23 |
| Piromelatine | 21, 22 |
| GABAergic system signaling | None | - |
| Adenosine receptor signaling | Caffeine | 19-22 |
| Histaminergic system signaling | None | - |



