| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website https://www.neurores.org |
Original Article
Volume 000, Number 000, December 2024, pages 000-000
Role of Serum Biomarkers in the Assessment of Traumatic Brain Injury
Michele Chiconea, e, Marinella Marrazzib, Adriana Paladinic, Giorgia Valentinid, Giambattista Lobreglioa
aClinical Pathology and Microbiology Unit, “Vito Fazzi” General Hospital, Lecce, Italy
bEmergency Department, “Vito Fazzi” General Hospital, Lecce, Italy
cNeuroradiology Unit, Vito Fazzi General Hospital, Lecce, Italy
dDepartment of Biological and Environmental Sciences and Technologies, University of Salento, Lecce, Italy
eCorresponding Author: Michele Chicone, Clinical Pathology and Microbiology Unit, “Vito Fazzi” General Hospital, Lecce 73100, Italy
Manuscript submitted November 11, 2024, accepted November 19, 2024, published online December 19, 2024
Short title: Serum Biomarkers in the Assessment of TBI
doi: https://doi.org/10.14740/jnr870
| Abstract | ▴Top |
Background: Each year, approximately 60 million people suffer a traumatic brain injury (TBI) of varying severity, classified as mild, moderate, or severe. Cranial computed tomography (CT) remains the primary imaging modality of choice for the diagnosis of intracranial lesions, such as hemorrhage or edema, in patients with TBI treated in the emergency department during the acute post-trauma period. CT, combined with patient symptoms and physical examinations, is essential to guide the care of these patients. However, this approach involves exposure to high doses of radiation and requires significant healthcare resources and costs. Furthermore, this diagnostic technique can reveal intracranial lesions in less than 10% of cases of mild-to-moderate TBI. For these reasons, there has been a strong and growing interest in more objective clinical methodologies in the identification of brain lesions, focusing attention on specific biomarkers, i.e. proteins present in the serum closely associated with TBI.
Methods: In this study, some blood biomarkers of TBI, including neuron-specific enolase (NSE), S100B, neurofilament light chain (NFL), ubiquitin C-terminal hydrolase L1 (UCH-L1) and glial fibrillary acidic protein (GFAP) were evaluated in patients who suffered head trauma of different severity, transported to the Emergency and Acceptance Department (D.E.A.) of the “Vito Fazzi” Hospital in Lecce between March 1, 2023 and September 1, 2023.
Results: Based on the diagnostic performances detected on the five tests taken into consideration, GFAP has therefore revealed itself as a potential biomarker to be used in emergency medicine. In fact, since it has not shown any cases of false negative, its high diagnostic sensitivity would allow, for serum values measured within 12 h of mild head trauma lower than the cut-off of 35 pg/mL, to exclude with a good safety margin, patients to be subjected to cranial CT.
Conclusions: Our results support GFAP as a biomarker with the highest negative predictive value in predicting the absence of TBI damage by selecting patients in the emergency department who could avoid performing CT. The application of this study would lead to a significant reduction in patient waiting times in the emergency department and a lower workload for the neuroradiology facility, a reduction in healthcare costs for instrumental investigations and would also avoid unnecessary radiation to the patient.
Keywords: Traumatic brain injury; Cranial computed tomography; Glial fibrillary acidic protein; Ubiquitin C-terminal hydrolase L1; Neurofilament light chain; Neuron-specific enolase; S100B protein; Glasgow coma scale
| Introduction | ▴Top |
Traumatic brain injury (TBI) refers to damage caused by a physical, mechanical incident involving any cranio-encephalic region, temporarily or permanently compromising brain function. It is important to note that cranial injury is not always synonymous with brain damage; it can result in skull fractures or complications involving the organs within the cranium. This phenomenon often arises from road accidents, falls, sports incidents, assaults, and other unforeseen events, affecting individuals of all ages.
Cranial trauma presents a significant and complex challenge in the fields of emergency medicine and neurology, posing both medical and social challenges. It can lead to permanent disability or even death, significantly impacting the quality of life for those involved and their families. Understanding the causes, pathophysiological mechanisms, and appropriate management strategies for cranial trauma is essential for improving clinical outcomes and reducing long-term consequences.
Types of cranial trauma
Cranial trauma can be classified based on the severity of the injuries and the presence of structural damage to the skull or brain. The most common classification is based on the Glasgow coma scale (GCS) and the presence of cranial fractures. The GCS is a widely used clinical tool to assess the consciousness level of a patient with brain injury or cranial trauma. It was developed in 1974 at the University of Glasgow, hence the name, and has become one of the standard instruments for evaluating the level of consciousness and the severity of brain injuries. The GCS evaluates three main components of patient responses: eye opening (eyes), verbal response (verbal), and motor response (motor) (Fig. 1).
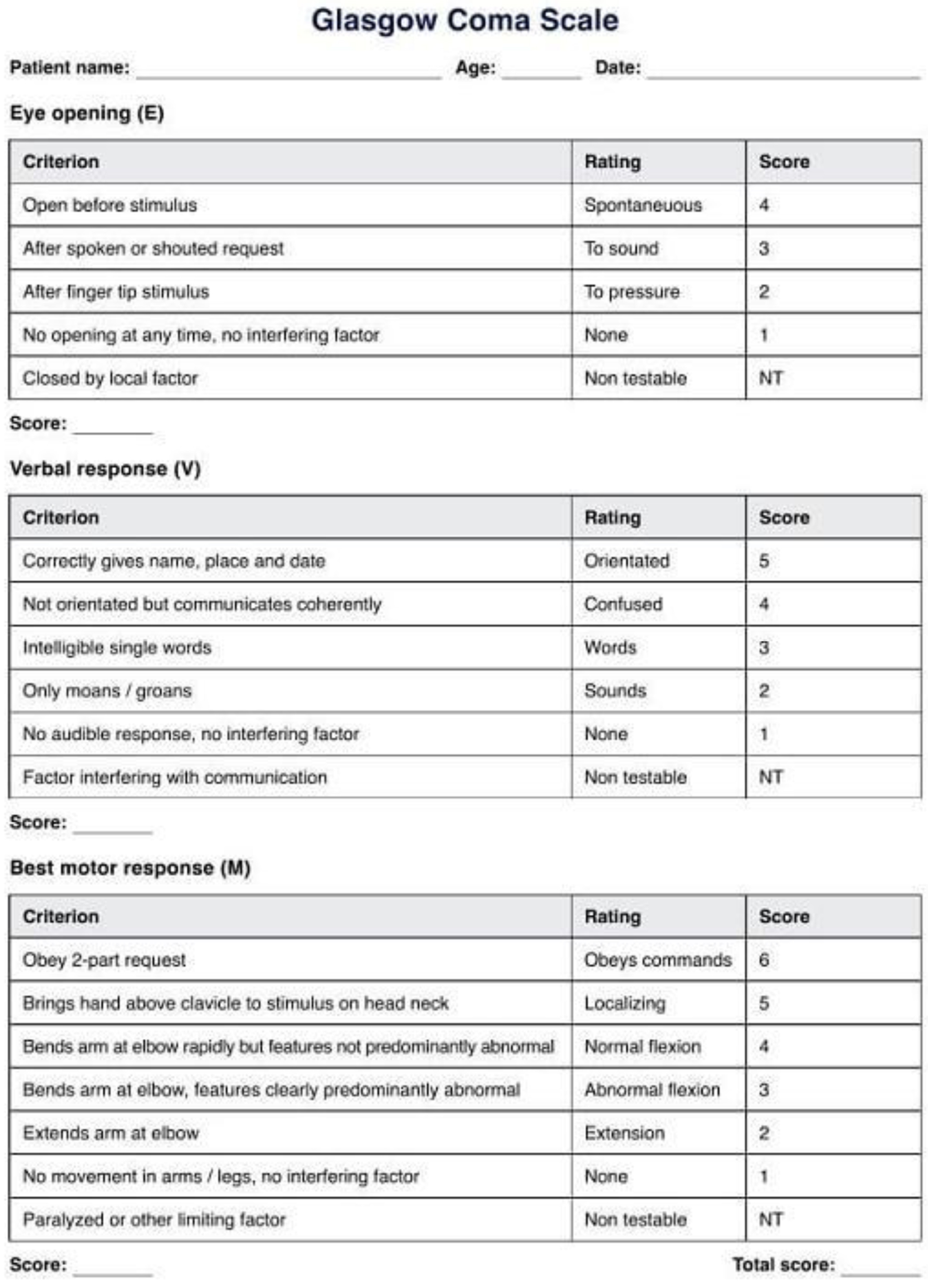 Click for large image | Figure 1. Glasgow coma scale. |
Each component is assessed on a numerical scale, and the total score is obtained by summing the scores of each component [1]. 1) Eye opening: The evaluation of eye response measures the degree of eye opening of the patient. A score from 1 to 4 is assigned, where 4 indicates spontaneous eye opening; 3 indicates eye opening in response to verbal command; 2 indicates eye opening in response to painful stimuli; 1 indicates no eye opening. 2) Verbal response: The assessment of verbal response measures the patient’s ability to speak and communicate. A score from 1 to 5 is assigned, where 5 indicates oriented and able to communicate coherently; 4 indicates confused but responding appropriately; 3 indicates speaking inappropriately, and responding with irrelevant words; 2 indicates making incomprehensible sounds; 1 indicates no verbal response. 3) Motor response: The evaluation of motor response measures the patient’s response to motor stimuli. A score from 1 to 6 is assigned, where 6 indicates obeys appropriate motor commands; 5 indicates localized response to pain (e.g., withdrawal from painful stimuli); 4 indicates flexor response to pain (e.g., flexion of the arm in response to painful stimuli); 3 indicates extensor response to pain (e.g., extension of limbs in response to painful stimuli); 2 indicates uncontrolled response to pain (nonspecific movements); 1 indicates no motor response.
The total GCS score ranges from a minimum of 3 (absence of responses) to a maximum of 15 (normal responses). A lower score indicates compromised consciousness and greater severity of brain injury.
This GCS score helps doctors classify cranial trauma into different severity categories [2]: 13 - 15 indicates mild cranial trauma; 9 - 12 indicates moderate cranial trauma; 3 - 8 indicates severe cranial trauma.
Symptoms of cranial trauma can vary depending on the severity of the injury and the area of the brain involved; some symptoms are immediately apparent, while others may not manifest until days or weeks after the injury. Consequently, it is possible to correlate the symptoms with the score obtained using the GCS.
With mild cranial trauma, the patient may remain conscious or may lose consciousness for a few seconds or minutes. The individual may also feel confused for some days or weeks after the initial injury.
Other symptoms include (cranial trauma, n.d.): headache; mental confusion; feeling of light-headedness; drowsiness; double vision, blurred vision, or tired eyes; ringing in the ears; bad tastes in the mouth; fatigue or lethargy; changes in sleep patterns; changes in behavior or mood; problems with memory, concentration, attention, or thinking.
Symptoms may regress or remain the same; worsening symptoms indicate progressive damage such as intracranial hemorrhage or the formation of cerebral edema, which, by blocking the flow of cerebrospinal fluid (CSF), leads to hydrocephalus, a sign and driver of more severe damage.
With moderate or severe cranial trauma, the patient may exhibit the same symptoms, associated with: loss of consciousness (vigilance); changes in personality; severe, persistent, or worsening headache; repeated vomiting or nausea; epileptic seizures; inability to wake up; mydriasis, i.e., dilation (increased diameter) or paralysis of one or both pupils; dysphasia: altered, slurred, incomprehensible speech; weakness, tingling, or numbness of the extremities; loss of coordination, and/or increased confusion, restlessness, or agitation; vomiting and neurological deficit (e.g., weakness in an arm or leg), together are important prognostic indicators and their presence requires immediate computed tomography (CT) and often also neurosurgical intervention.
Young children with moderate or severe cranial trauma may exhibit some of the preceding signs, as well as others specific to children, such as persistent crying, inability to be comforted, and/or refusal to drink, breastfeed, or eat.
Biomarkers for TBI
For a protein biomarker for TBI based on biological fluids to be clinically useful, it should ideally have as many of the following attributes as possible.
Type of biological fluid and accessibility
The biomarker should be easily measurable in accessible biological fluids such as CSF, serum, plasma, and/or whole blood.
For severe TBI in neuro-intensive care, biomarker detection should be possible in CSF, serum, plasma, and/or whole blood.
For moderate and mild TBI in emergency departments or non-intensive care settings, the biomarker should be detectable in serum, plasma, and/or whole blood for rapid and convenient access.
High levels after TBI
The biomarker should show elevated levels in various forms and severities of human TBI in the acute phase (3 - 24 h after injury), compared to uninjured healthy controls.
It must have low baseline levels in the biological fluids of the healthy control population.
Origin and nature of the biomarker
The biomarker should primarily originate from the damaged brain.
It should be sensitive to the severity of TBI, as defined by indices such as the GCS and abnormalities in CT scans.
Detection methods
Biomarker levels should be easily determinable and quantifiable in the aforementioned biological fluids using methods such as sandwich enzyme-linked immunosorbent assay (ELISA) or similar immunological assays, with at least two assay formats or platforms available.
Analysis platforms should meet acceptable reliability and test-retest reproducibility requirements for FDA regulations.
Translational validity and preclinical evidence
The biomarker should demonstrate similar profiles in the biological fluids of at least two different animal models of cranial trauma, such as penetrating ballistic brain injury (PBBI) or explosive blast overpressure brain injury (OBI).
Correlation with clinical outcomes and treatments
Acute initial levels of the biomarker (within the first 48 h after injury) should correlate with commonly accepted outcome indices of TBI patients, such as the Glasgow outcome scale (GOS) or GOS-extended (GOS-E).
Biomarker levels in post-TBI biological fluids should respond to therapeutic treatments.
These requirements represent an overview of the key criteria that a protein biomarker for TBI should meet to be clinically useful and significant. Reflecting the various pathophysiological alterations that occur during cranial trauma, a panel of protein biomarkers detectable in biological fluids have recently been identified. This biomarker panel covers a range of processes, including axonal damage, dendrite injuries, neuronal cell body damage, demyelination, synaptic damage, and responses activated by microglial cells (Fig. 2) [3].
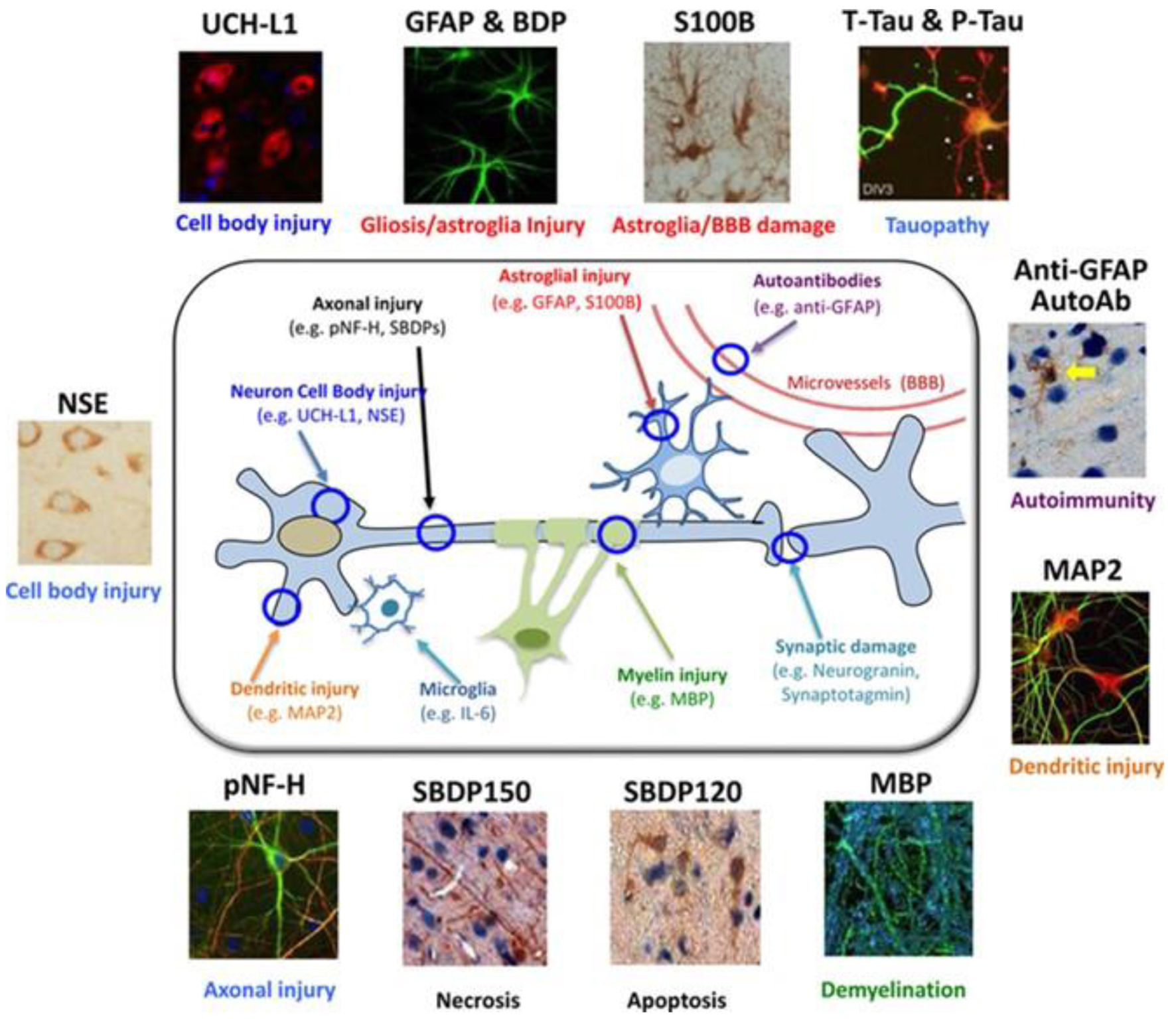 Click for large image | Figure 2. Graphical representation of the main protein biomarkers of cranial trauma associated with different pathophysiological processes in cranial trauma. |
In this study, some aspects related to the following markers will be explored: neuron-specific enolase (NSE), ubiquitin C-terminal hydrolase L1 (UCH-L1), glial fibrillary acidic protein (GFAP), calcium-binding protein of astroglial origin (S100B), and neurofilament light chain (NFL) [4].
Neuronal cell body injury markers
NSE
The enzyme NSE is recognized as a particular isoform of a glycolytic enzyme [5]. In vertebrates, there are three variants of the enolase enzyme, each produced by distinct genes: enolase α is widespread throughout the body, enolase β is specific to muscles, and enolase γ is specific to neurons. It has been established that the NSE enzyme enables obtaining quantitative data on brain injuries and/or contributes to improving diagnosis and assessment of evolution in cases of ischemic stroke, intracerebral hemorrhage, episodes of seizures, patients in a comatose state after cardiopulmonary resuscitation following cardiac arrest, and traumatic brain injuries [6, 7]. Elevated concentrations of NSE in the bloodstream have been recorded following severe cranial injuries. Increased NSE concentrations have also been observed in cases of mild traumatic brain injury (mTBI). One of the main drawbacks associated with using NSE as a specific indicator for cranial injuries is the fact that this enzyme is abundantly present in red blood cells as well. This evidence has led researchers to consider a correction for hemolysis when measuring NSE concentration in blood [3].
UCH-L1
The enzyme UCH-L1 is a protein mainly located in the cytoplasm of neuronal cell bodies. It has been identified as one of the potential biomarkers of TBI in recent proteomic studies [8]. Recent research has also highlighted the usefulness of UCH-L1 in long-term prediction of severe cranial injuries. Additionally, two separate studies have revealed the presence of UCH-L1 in the serum or plasma of individuals with mTBI.
Astroglial biomarkers
GFAP
GFAP is emerging as the most reliable biomarker for TBI [9]. It has also been suggested that UCH-L1 and GFAP protein could form the basis of a biomarker panel representing the two main cell types present in the brain [3]. GFAP levels increase within a time frame of 3 - 34 h in CSF, as well as in serum/plasma, following severe cranial injuries. The same is observed in serum and plasma samples in cases of cranial trauma from moderate to mild TBI.
GFAP, as a biomarker, is mainly released from damaged brain tissue in the form of intact GFAP protein (50 kDa) or its degradation products (GFAP-BDP; 44 - 38 kDa) in biological fluids such as CSF and serum/plasma shortly after a cranial trauma. Alongside studies conducted on human cranial injuries, increases in GFAP levels have been recorded in CSF in various severe cranial trauma animal models, as well as in serum/plasma samples in mTBI models. Evidence indicates that the increase in GFAP after TBI is correlated with the severity of the injury. Additionally, GFAP levels are associated with pathological changes in CT images and the clinical course of patients [3].
S100B
S100B protein is an 11 kDa protein that binds calcium of astroglial origin. It is one of the most widely investigated biomarkers for brain injuries to date [10, 11]. Data from preclinical models of cranial injuries in animals regarding S100B exist. This biomarker has been studied in various cases of TBI of different severities. However, it should be noted that S100B can also be released by adipose tissue and cardiac/skeletal muscles [12-14], and therefore, an increase in its levels may occur even in the presence of injuries not involving the skull. Despite these confounding factors, S100B does indeed represent a sensitive biomarker for predicting abnormalities in CT images and assessing the development of post-concussion syndrome among patients with mTBI [3].
Delayed axonal injury and demyelination markers
NFL
Neurofilaments (NFs) are considered “class IV” intermediate filaments with a diameter of approximately 10 nm and are exclusively present in neurons. These filaments form bundles known as neurofibrils and constitute a fundamental component of the cytoskeleton, whose main role is to provide structural support to the axon and regulate its diameter. NFs are composed of three different polypeptide subunits in terms of molecular weight: NFL (68 kDa), neurofilament medium chain protein (NFM; 150 kDa), and neurofilament heavy chain protein (NFH; 200 kDa). After a proteolysis process, these subunits can be released from the cytoskeleton into the cytosol or, in some cases, into the extracellular fluid, especially if the integrity of the cell membrane is compromised. Increased NFL levels in the serum have been observed in individuals with cranial trauma [15]. It is important to note that the release of NF proteins into biological fluids is a delayed process compared to the initial event of the injury, occurring in the days following. Consequently, it may reflect ongoing axonal degeneration and could be correlated with cognitive decline and progression in patients with chronic cranial trauma [3].
Purpose of the study
Over the last decade, significant scientific advances have expanded our understanding of the complex and variable pathophysiological processes related to TBI. Annually, approximately 60 million people experience cranial trauma of varying severity. Cranial CT is the preferred investigation for evaluating patients with TBI; however, it exposes the individual to high doses of radiation and consumes significant healthcare resources. For these reasons, and considering that CT detects intracranial lesions in less than 10% of subjects with mild to moderate TBI on average, clinical decision rules have been developed to reduce unnecessary CT scans. Nevertheless, these rules have had only a modest impact on the utilization of the investigation. The search for more objective clinical methods to detect brain injuries has sparked growing interest in certain biomarkers, such as proteins present in the serum closely associated with TBI. In this study, attention has been focused on some TBI biomarkers, such as S100B, GFAP, UCH-L1, NSE, and NFL, to conduct a preliminary evaluation of patients with mild to moderate TNI. The aim of this study was to assess the performance of these biomarkers, verifying their accuracy and correlation with radiological findings in the context of a possible “negative predictive value” (NPV). This aspect, if consolidated, would potentially allow limiting the systematic use of CT scans for mild cranial trauma, where the results of the same biomarkers prove to be physiological. Better selection of patients requiring CT scans would indeed lighten the burden of requests to radiology departments, reducing waiting times in emergency medicine, significant savings both in terms of healthcare resource optimization and reduction of unnecessary radiation exposure for patients. The integration of these innovative laboratory data into the overall management of patients with TBI would allow, following the definition of specific protocols and operational procedures, a faster, appropriate, and simplified diagnostic pathway for emergency departments and urgent care.
Study design and case series
For this investigation, an observational study was conducted on a sample of patients admitted to the Emergency and Admission Department (E.A.D.) of the “Vito Fazzi” Hospital in Lecce via the emergency room between March and September 2023, following cranial traumas of various severities. For this study, in agreement with the directors of the Department of Emergency Medicine and Surgery and the Complex Operating Unit of Clinical Pathology and Microbiology, an informed consent form was formulated to obtain authorization for venous sampling and analysis of new biomarkers associated with cranial trauma in selected patients.
This form contains clinical and anamnestic information regarding the patients’ clinical status, including past medical history, severity of the head trauma, presence of concurrent pathologies, and any use of antiplatelet or anticoagulant medications.
In the absence of family members or if the patient is unable to provide consent directly, consent signed by the emergency room physician responsible for managing the patient was requested.
The study design primarily focused on cases of moderate to mild head trauma, as one of the main objectives of the research was to identify a potential NPV among the new biomarkers tested to select the category of patients for whom a CT scan might not be necessary. However, the decision to include patients with severe trauma in the case series was solely driven by the need to assess the diagnostic reliability of all five laboratory tests in demonstrating critical values correlated with the extent of injury. Based on this decision, if any of these tests had not shown pathological results related to severe damage, even in just one of the patients considered, the same test would have been preemptively excluded from the study as unreliable.
| Materials and Methods | ▴Top |
This study was conducted in accordance with the ethical standards of the responsible institution on human subjects and with the Declaration of Helsinki. No prior approval by the ethics committee was required since the serum samples used for the study, for which an informed consent form was prepared, were derived from residual material from the collection officially requested for its clinical use by the emergency room doctors of the health care institution.
Biomarkers evaluated in the study
In this study, specific serum analyses were conducted to evaluate the expression of certain biomarkers in patients with TBI. Serum assays performed on the control group of healthy subjects included UCH-L1 and GFAP biomarkers to redefine the laboratory’s reference values compared to those provided by the manufacturer, and NFL assays to establish cutoffs and reference intervals for the healthy population compared to TBI patients. This choice was guided by the fact that reference values for the classic biomarkers, S100 and NSE, have long been established in many studies in the recent scientific literature and therefore did not require further analysis. To obtain biological samples, blood draws were performed within the first 12 h after the traumatic event. Serum samples were then centrifuged at a speed of 4,000 rpm for 10 min to separate solid components from liquid ones. The resulting aliquots were frozen in 1.5 mL Eppendorf tubes and stored at a temperature of -80 °C until they were analyzed for the biomarkers of interest. This approach allowed for obtaining high-quality biological samples and conducting accurate and reliable analyses to assess the expression of biomarkers in patients with TBI and in healthy control subjects.
Immunological test for NSE
The immunological test for quantitative determination of NSE in human serum was conducted using the clinical biochemistry analytical platform “Roche Cobas series 8000”, utilizing the commercial kit “Roche Elecsys NSE”. The reference values applied in our laboratory for serum NSE assay range from 0 to 17 µg/L in the healthy population. The “Cobas” instruments are a series of automated chemical analyzers produced by Roche Diagnostics S.p.A., one of the leading companies in the field of in vitro diagnostics and healthcare. These instruments are used in clinical analysis laboratories to conduct diagnostic tests on a variety of biological samples, such as blood, serum, plasma, urine, CSF, and other biological fluids. The term “Cobas” is an acronym for “combined analysis and storage” and has been used to describe the capability of these instruments to perform a range of tests and efficiently store patient data. The analytical unit used for the test execution is the Cobas e 801, a high-throughput immunochemistry module that performs a wide range of heterogeneous immunological tests using the electrochemiluminescence immunoassay (ECLIA) technology.
Immunological test for UCH-L1 and GFAP
The TBI test is a chemiluminescent microparticle capture immunodiagnostic assay (CMIA) panel used for quantitative measurements of GFAP and UCH-L1 in human plasma and serum samples, providing a semiquantitative interpretation of analytical results derived from these measurements using the Abbott s.r.l. Alinity i analytical platform, which supplied the UCH-L1 and GFAP commercial kits.
The GFAP and UCH-L1 assays use a logistic curve fitting data processing method with a four-parameter logistic curve (4PLC, weighted Y) to generate a calibration curve and results.
The assay cutoffs established by the manufacturer are 35.0 pg/mL (35.0 ng/L) for GFAP and 400.0 pg/mL (400.0 ng/L) for UCH-L1.
GFAP and UCH-L1 results are reported separately, and the software provides an interpretation of the TBI test based on their respective cutoff values.
Immunological test for S100
The immunological test for the quantitative determination of S100 proteins in human serum was conducted using analysis instrumentation from DiaSorin s.p.a., a company specialized in providing instruments and reagents for laboratory testing. The analytical platform used for this analysis is the Liaison XL system, known for its ability to perform a variety of immunochromatographic tests, including those based on chemiluminescence technology.
The kit provided by DiaSorin is the Liaison S100, a chemiluminescent sandwich immunoassay (CLIA), which could be utilized to measure the concentration of S100 protein in serum or plasma samples, relevant in medical contexts for the diagnosis or monitoring of neurological conditions such as TBI, or in oncology for monitoring certain types of tumors. The reference values for serum S100 assay applied in our laboratory have a cutoff < 0.15 µg/L for the healthy population.
Immunological test for NFL
The immunological test for the quantitative determination of NFL in human serum was conducted using the Lumipulse G600II analysis platform from Fujirebio s.r.l., which provided the Lumipulse G NFL Blood kit.
Fujirebio is a Japanese company specialized in in vitro diagnostics, namely in the production of diagnostic tests used to analyze biological samples such as blood, urine, or tissues.
The Lumipulse G600II analytical unit used for the test execution is an analysis system that comprises a series of immunoassay reagents for the quantitative measurement of NFL in plasma or serum samples.
The Lumipulse G600II assay principle utilizes chemiluminescent enzyme immunoassay (CLEIA) technology to detect and quantify NFL in biological samples.
The NFL calibrator and blood sample are added to a solution containing special particles. The NFL present in the sample specifically binds to the anti-NFL monoclonal antibody that is immobilized on the particles. The particles are then washed and rinsed carefully to remove any unbound or interfering material. Monoclonal antibodies against NFL labelled with alkaline phosphatase (ALP) (mouse) are then added specifically to the immunocomplexes formed previously on the particles, creating further antigen-antibody complexes. The particles are again washed and rinsed to remove any unbound or interfering material.
A solution containing a chemiluminescent substrate (AMPPD*) is added to the particles and mixed. The AMPPD is dephosphorylated, thanks to the catalytic action of the ALP, conjugated to the particles. Luminescence is generated as a result of the cleavage reaction of the dephosphorylated AMPPD.
This reaction produces light at a maximum wavelength of 477 nm. The intensity of the luminescent signal directly reflects the quantity of NFL present in the sample.
Statistical analysis
For the statistical analysis of serum assays performed on TBI patients, a cross-correlation analysis between the five different biomarkers was conducted using the Student’s t-test with statistically significant values of P value < 0.05 using Excel 2013 (Microsoft) software. For NFL, considering that there are few studies in the literature regarding reference values in healthy subjects to date, we decided to use the same control group to define a reference range in the healthy population, verifying the accuracy, specificity, and diagnostic sensitivity of the method by producing a receiver operating characteristic (ROC) curve using MedCalc statistical software version 19.9.1 (MedCalc Software Ltd, Ostend, Belgium). The correlation study between two variables, in our case NFL levels and patient age, considered the Pearson index with moderate correlation intervals greater than 0.3 and less than 0.7 and strong correlation with Pearson indices greater than 0.7.
| Results | ▴Top |
Study population
During the internship period conducted at the analysis laboratory of the “Vito Fazzi” Hospital in Lecce, between March 1, 2023 and September 1, 2023, a total of 68 patients with accidental TBI were enrolled (Table 1), of which 63 (93%) were mild, two (3%) were moderate, and three (4%) were severe in severity (Fig. 3).
 Click to view | Table 1. List of the 68 Total Cases of Traumatic Brain Injury Studied |
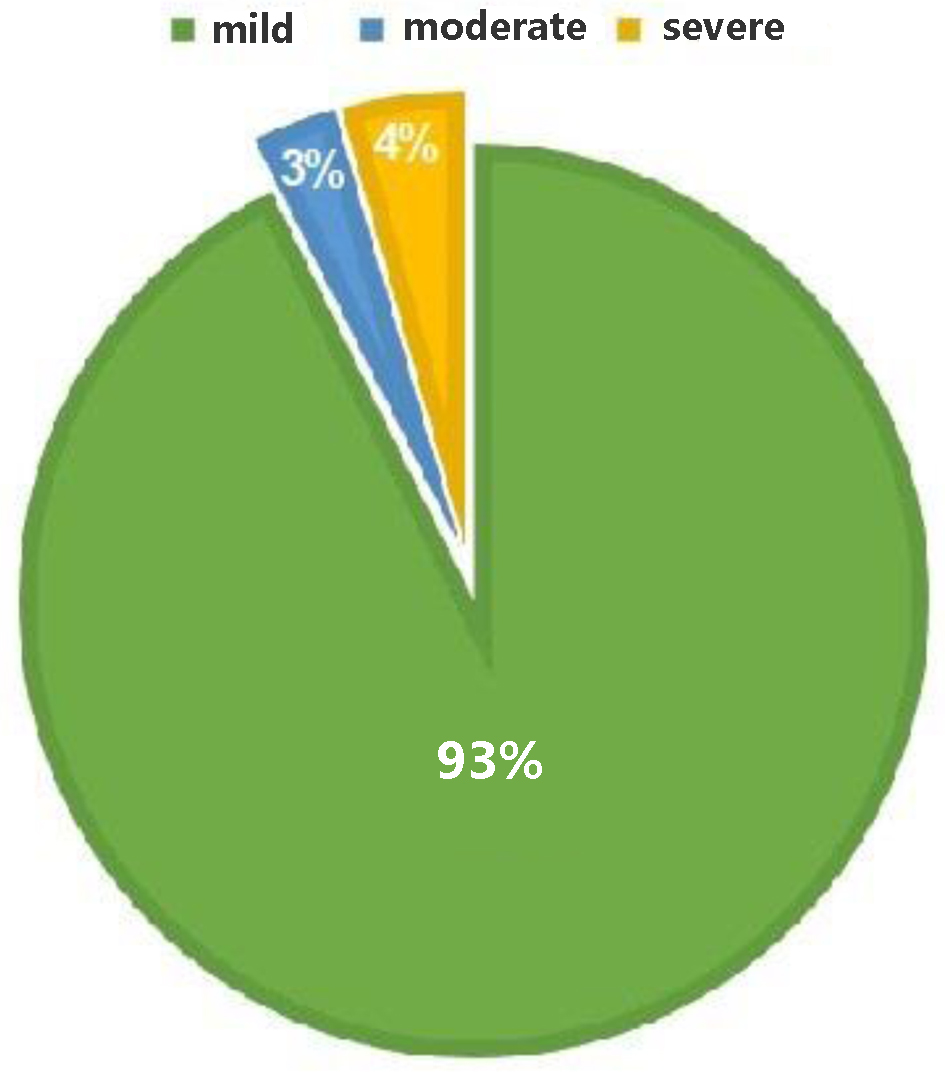 Click for large image | Figure 3. Distribution of cranial injuries and severity of damage among the examined population. |
Of these patients, one was under 6 years old, and therefore, consent for sample collection was granted by the parents. The others were mostly aged over 70 years. The distribution of the population by age groups (Fig. 4) showed that one patient was under 10 years old, two were aged between 10 and 29 years, nine (13%) between 30 and 49 years, 13 (19%) between 50 and 69 years, and 43 (68%) between 70 and 100 years; the mean age was 70 years.
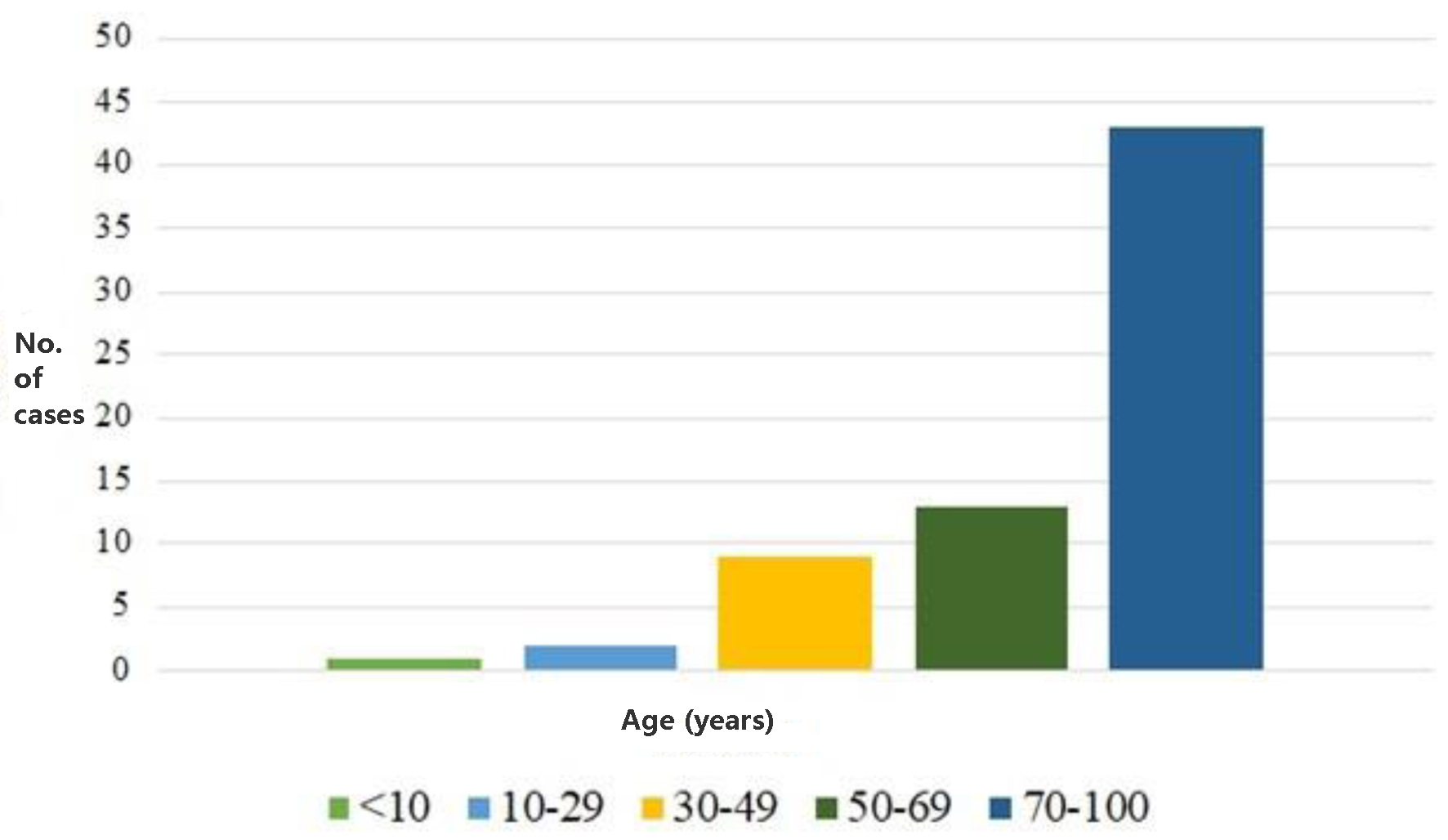 Click for large image | Figure 4. Distribution of the studied population related to age. |
In order to avoid false positives, five out of the 68 patients were excluded because their medical history revealed neurological disorders: one subject had a history of epilepsy, two subjects presented with dementia, while the remaining two had diagnoses of Alzheimer’s disease and Schwannoma. This decision was made to prevent potential overestimation of false positives in the assessment of serum biomarkers’ diagnostic performance due to the accumulation of molecules in the bloodstream resulting from chronic damage to the central or peripheral nervous system, rather than acute damage as the focus of this study.
Furthermore, out of the 68 patients with cranial trauma, 25 reported a history of anticoagulant or antiplatelet therapy; of these, nine subjects (36%) showed a positive CT scan. In the remaining 43 patients, the CT scan was positive in 10 cases (30%), suggesting that anticoagulant or antiplatelet therapy does not significantly increase the potential susceptibility to hemorrhagic events.
NSE assay
Out of the 68 patients with cranial trauma, plasma samples from only 29 were successfully obtained for subsequent analysis. After excluding five patients with various neurological disorders, the samples available for analysis further reduced to 24.
The results of the NSE assay (Table 2) highlight that, despite the majority of patients having mild injuries, the marker values often exceeded the threshold value of 17 µg/L. Specifically, 12 samples were positive, while two samples had values close to the threshold.
 Click to view | Table 2. NSE Marker Results Associated With Acute Cranial Trauma |
Furthermore, in the single case classified as severe, the test did not show a parallel increase in the marker (sample no. 114).
A more detailed analysis, comparing the assay results with the outcomes of the CT scan used as a diagnostic reference point, revealed that only six samples corresponded to true positives (TP). On the other hand, true negatives (TN) represent cases where a diagnostic test correctly identified that an individual does not have the condition of interest.
The analysis of the performance of the NSE test for TBI, with a cutoff value of 17 µg/L, showed an overall accuracy of 71% and a diagnostic specificity of 65%. However, there is an observed diagnostic sensitivity of 85%, accompanied by an NPV of 92% and a low positive predictive value (PPV) of 50% (Fig. 5).
 Click for large image | Figure 5. Scatter plot of NSE test performance. NSE: neuron-specific enolase. |
Examining these data through the ROC curve (Fig. 6), generated using statistical analysis conducted with the assistance of MedCalc software, it was possible to highlight a decrease in both sensitivity and specificity, with limited statistical significance.
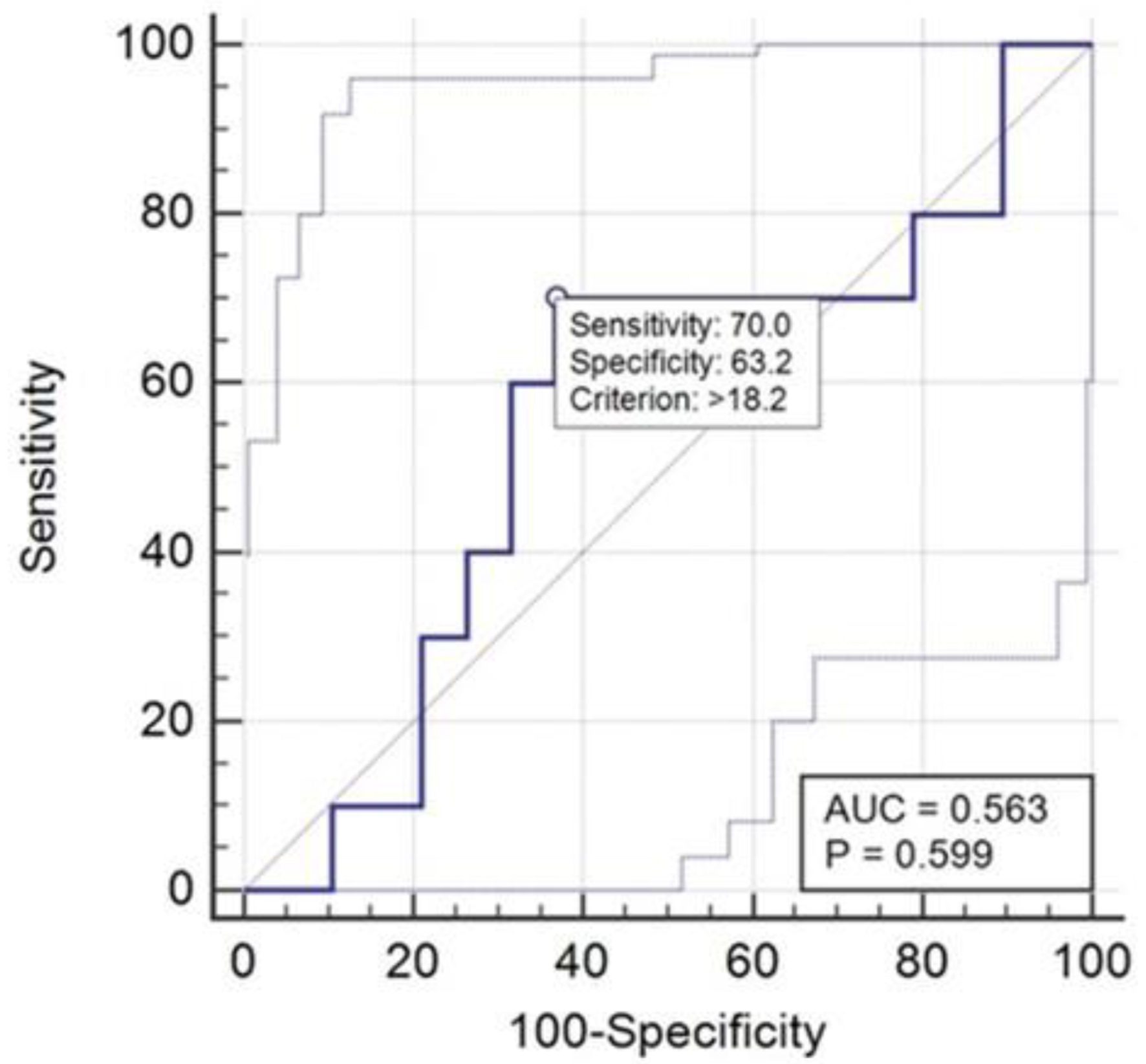 Click for large image | Figure 6. ROC curve related to NSE results. ROC: receiver operating characteristic; NSE: neuron-specific enolase. |
UCH-L1 and GFAP assays
The UCH-L1 test is provided by Abbott in association with the GFAP test as a single diagnostic panel, called the TBI kit, to be used on the Alinity i analyzer. In this case, out of the initial pool of 68 samples, it was possible to perform the test on only 59 samples, not only due to the exclusion of the five patients with alterations to the central nervous system but also due to limitations related to the depletion of the material to be examined.
The results regarding the UCH-L1 assay (Table 3) showed that, despite the majority of patients having mild damage, the marker values often exceeded the threshold value of 400 pg/mL. Specifically, 34 samples were positive, while only one sample had a value close to the threshold. Additionally, out of the three cases classified as severe, the test showed a concomitant increase in the marker level in only two patients (samples no. 18 and no. 19); even in the case classified as moderate, positivity was found (sample no. 26).
 Click to view | Table 3. UCH-L1 Marker Results Associated With Traumatic Brain Injury |
A detailed analysis, comparing the assay results with the outcomes of the CT scan, revealed that only 12 samples were TP, while 19 were TN. The results of the GFAP assay (Table 4) showed that the majority of cases classified as mild damage exceeded the threshold value of 35 pg/mL. The assay results were also altered in the three cases classified as severe (samples no. 18, no. 19, and no. 114) and in the single case analyzed classified as moderate (sample no. 26).
 Click to view | Table 4. GFAP Marker Results Associated With Traumatic Brain Injury |
Analysis of the assay results based on CT scan outcomes identified 17 TP samples. Comparing the two serum markers UCH-L1 and GFAP allowed to highlight that the two tests have comparable accuracy values (54% and 50%, respectively). However, the specificity was higher for UCH-L1, with a value of 48%, compared to the value of 31% obtained in the case of GFAP. However, the most substantial difference between the two tests was found in sensitivity. While the UCH-L1 test has low sensitivity (70%) and low NPV (80%), GFAP remains the better biomarker in terms of sensitivity (100%) and NPV (100%) for our study (Fig. 7).
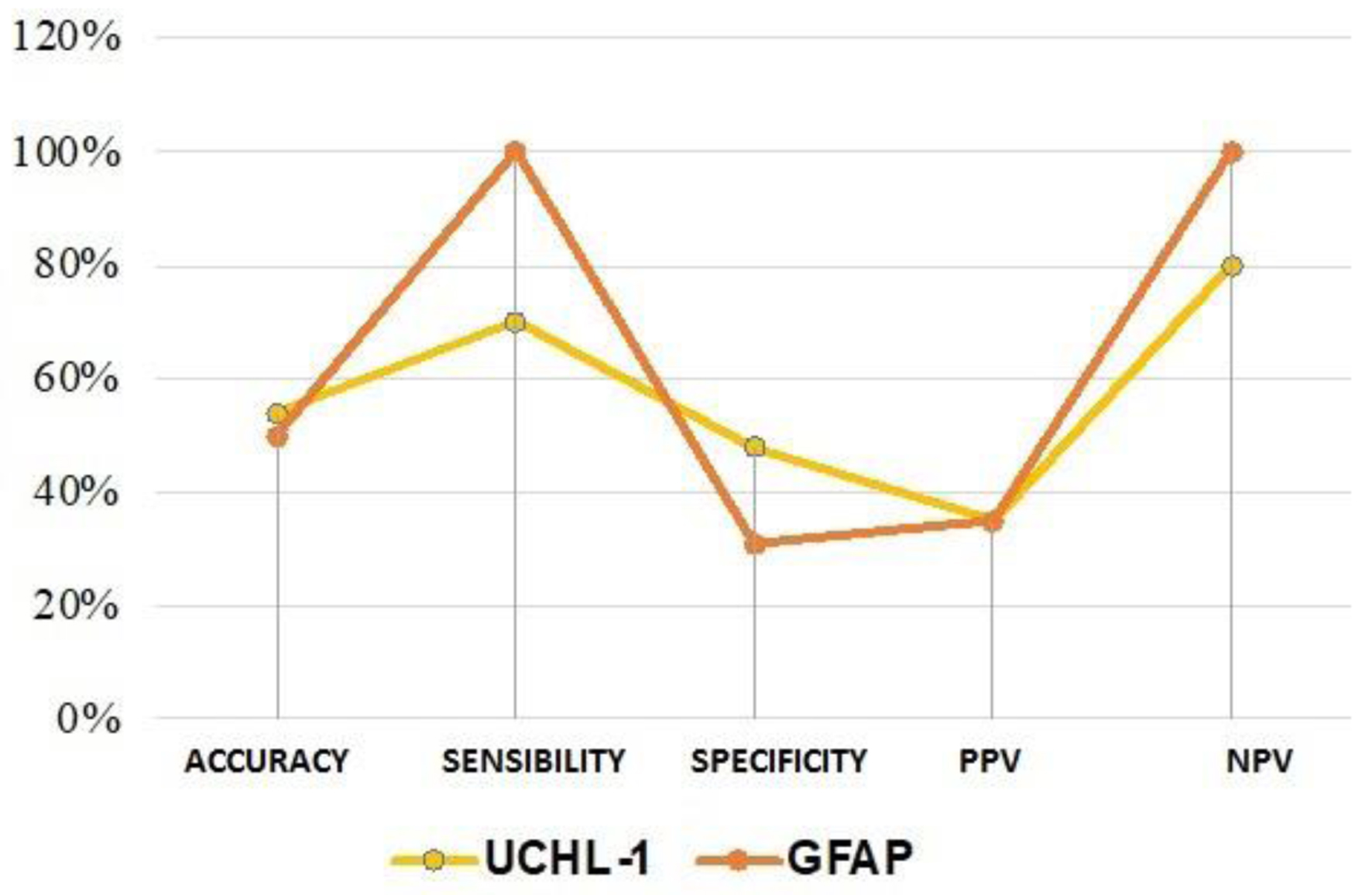 Click for large image | Figure 7. Comparison of the performance of UCH-L1 and GFAP tests. GFAP: glial fibrillary acidic protein; UCH-L1: ubiquitin C-terminal hydrolase L1. |
The analysis conducted through the ROC curve (Fig. 8), performed including the control group, showed for UCH-L1 a further reduction in sensitivity (61%), favoring an increase in specificity (71%), identifying a cutoff of 501.3 pg/mL, higher than the 400 pg/mL recommended by the manufacturer.
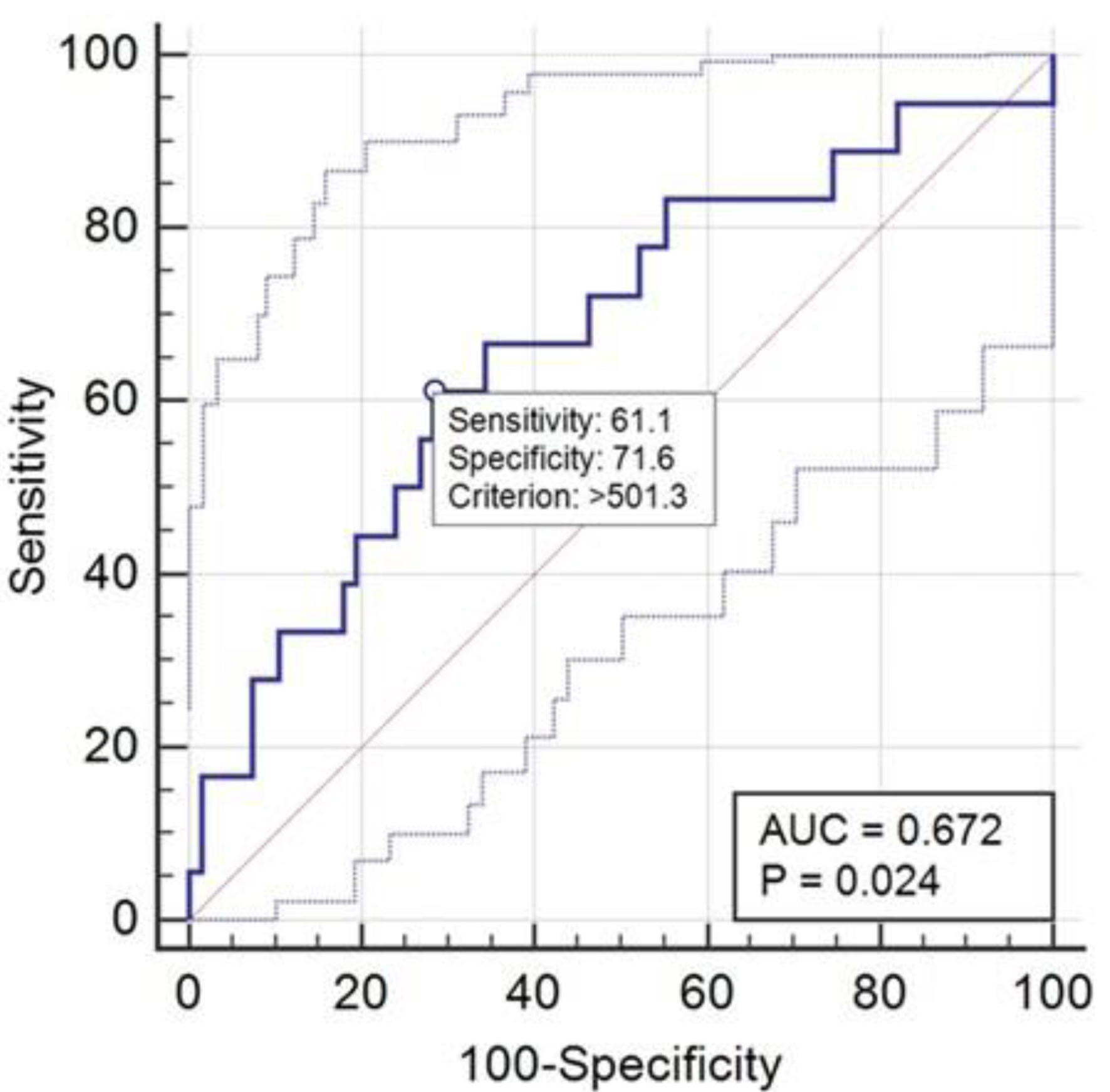 Click for large image | Figure 8. ROC curve for the performance of UCH-L1. ROC: receiver operating characteristic; UCH-L1: ubiquitin C-terminal hydrolase L1. |
The GFAP assay was the only test that showed 100% sensitivity and 100% NPV. This means that in all patients where the CT scan showed signs of intracranial lesions, this marker was higher than the cutoff value recommended by the manufacturer (35 pg/mL). Relative to the parameters calculated in this study, the analysis conducted using the ROC curve (Fig. 9a), including the control group, confirmed high sensitivity (94%) and increased specificity (63%), identifying a cutoff value of 63 pg/mL, higher than the one recommended by the manufacturer.
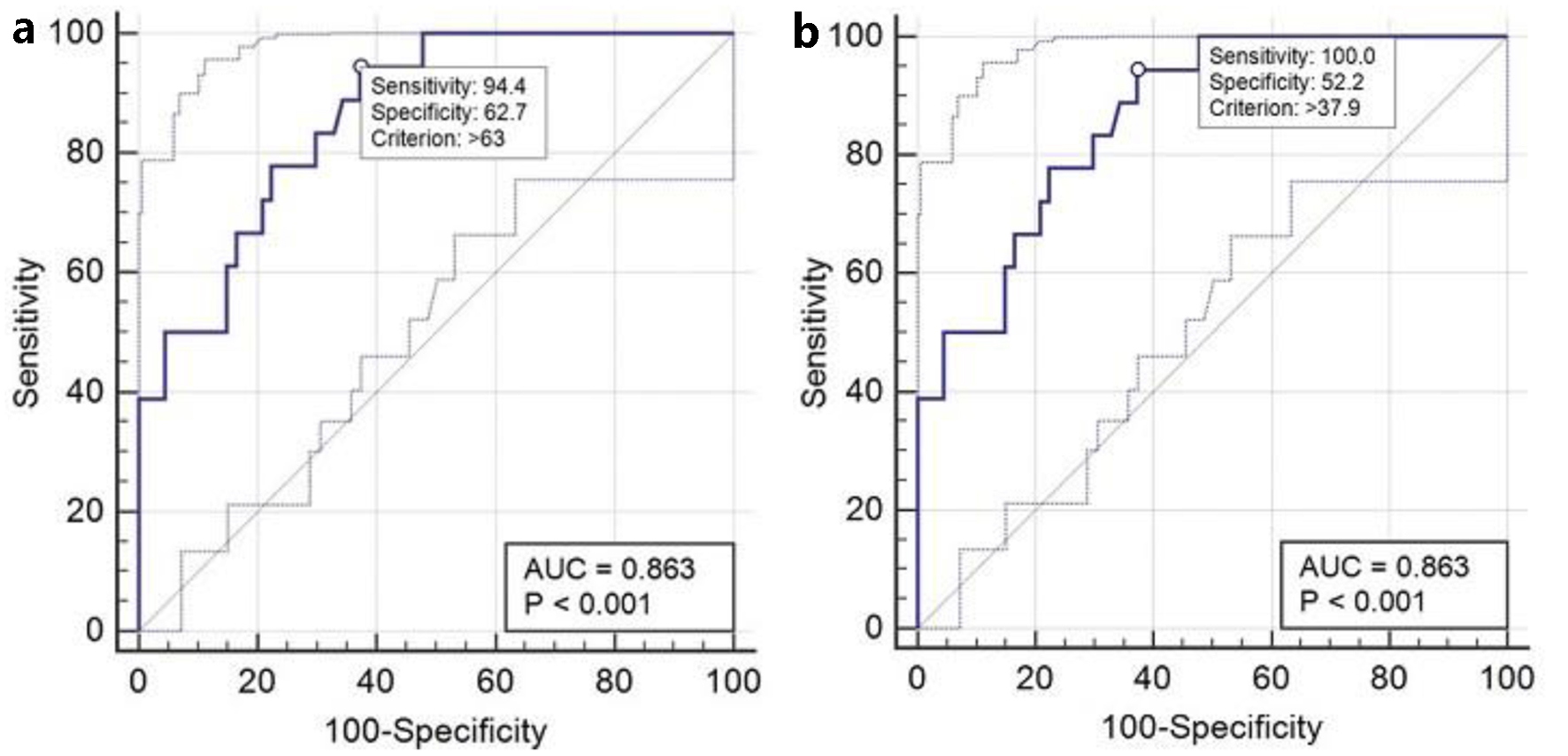 Click for large image | Figure 9. (a) ROC curve for the performance of GFAP. (b) ROC curve for the performance of GFAP with 100% sensitivity. GFAP: glial fibrillary acidic protein; ROC: receiver operating characteristic. |
Then, selecting a point on the ROC curve obtained where the sensitivity is 100% as calculated in this study, the software resized the cutoff to 37.9 pg/mL, very close to the value of 35 pg/mL suggested by the company, thus confirming the high sensitivity and diagnostic performance of the test (Fig. 9b).
S100 assay results
The results of the S100 assay (Table 5), conducted on 59 samples, also showed marker values above the threshold (0.15 µg/L) in 41 samples from subjects with mild classified cranial trauma. Moreover, the analysis detected alterations in the result in the three cases classified as severe (samples no. 18, no. 19, and no. 114) and in the only case analyzed classified as moderate (sample no. 26).
 Click to view | Table 5. S100 Marker Results Associated With Acute Cranial Trauma |
From the comparison between the assay results and the outcomes of the TAC, used as a diagnostic reference point, it emerged that only 16 samples were TP.
The analysis of the performance of the S100 test (Fig. 10) for TBIs, with a cutoff of 0.17 µg/L, revealed low accuracy (56%) and reduced diagnostic specificity (40%). However, it is possible to highlight a diagnostic sensitivity of 94%, accompanied by an NPV of 94% and a low PPV of 39%.
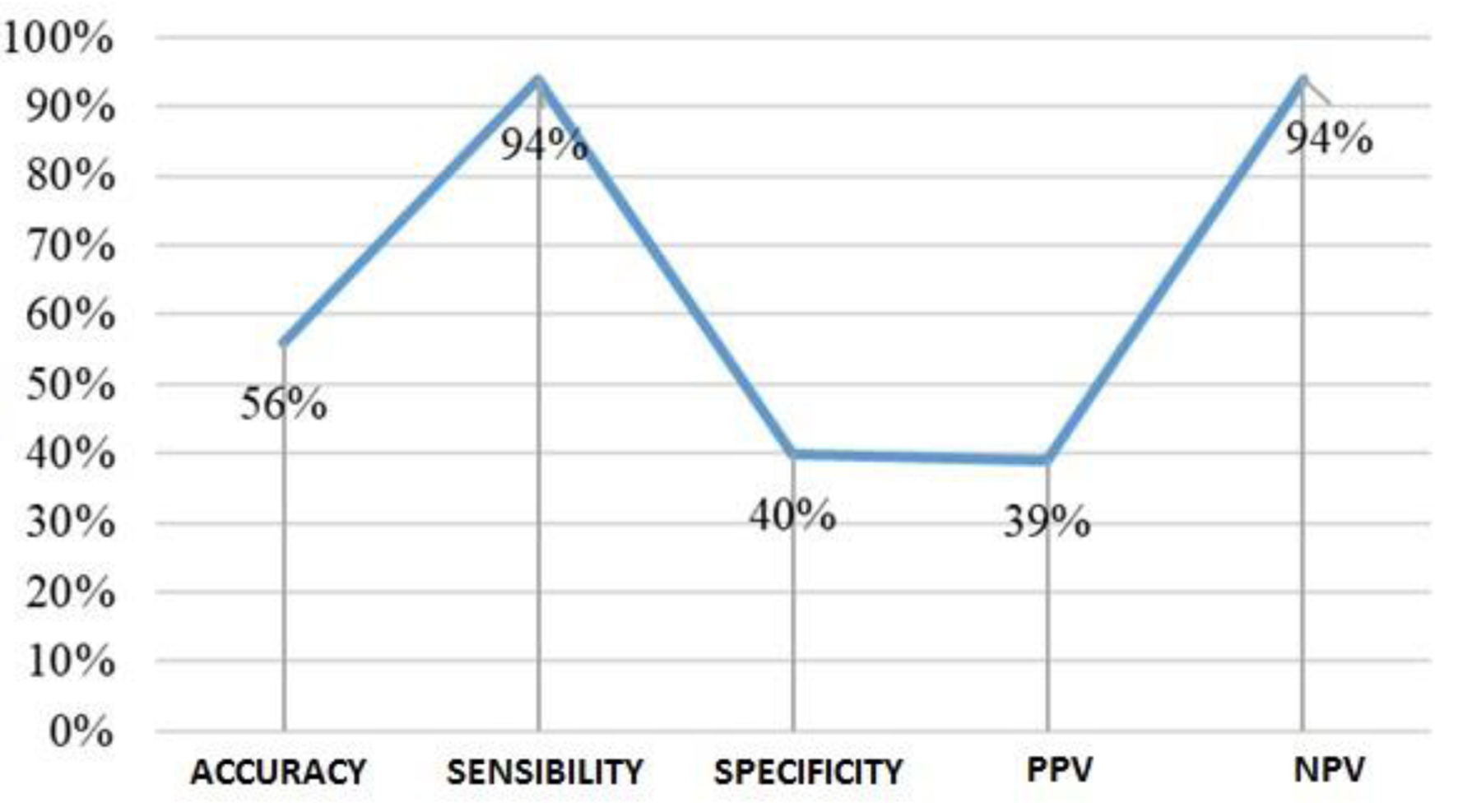 Click for large image | Figure 10. Scatter plot of S100 performance. |
Relative to the parameters calculated in this study, the analysis conducted through the ROC curve (Fig. 11) confirmed a slight reduction in sensitivity and specificity with a cutoff of 0.18 µg/L, a value very close to the 0.15 µg/L used in diagnostic routine.
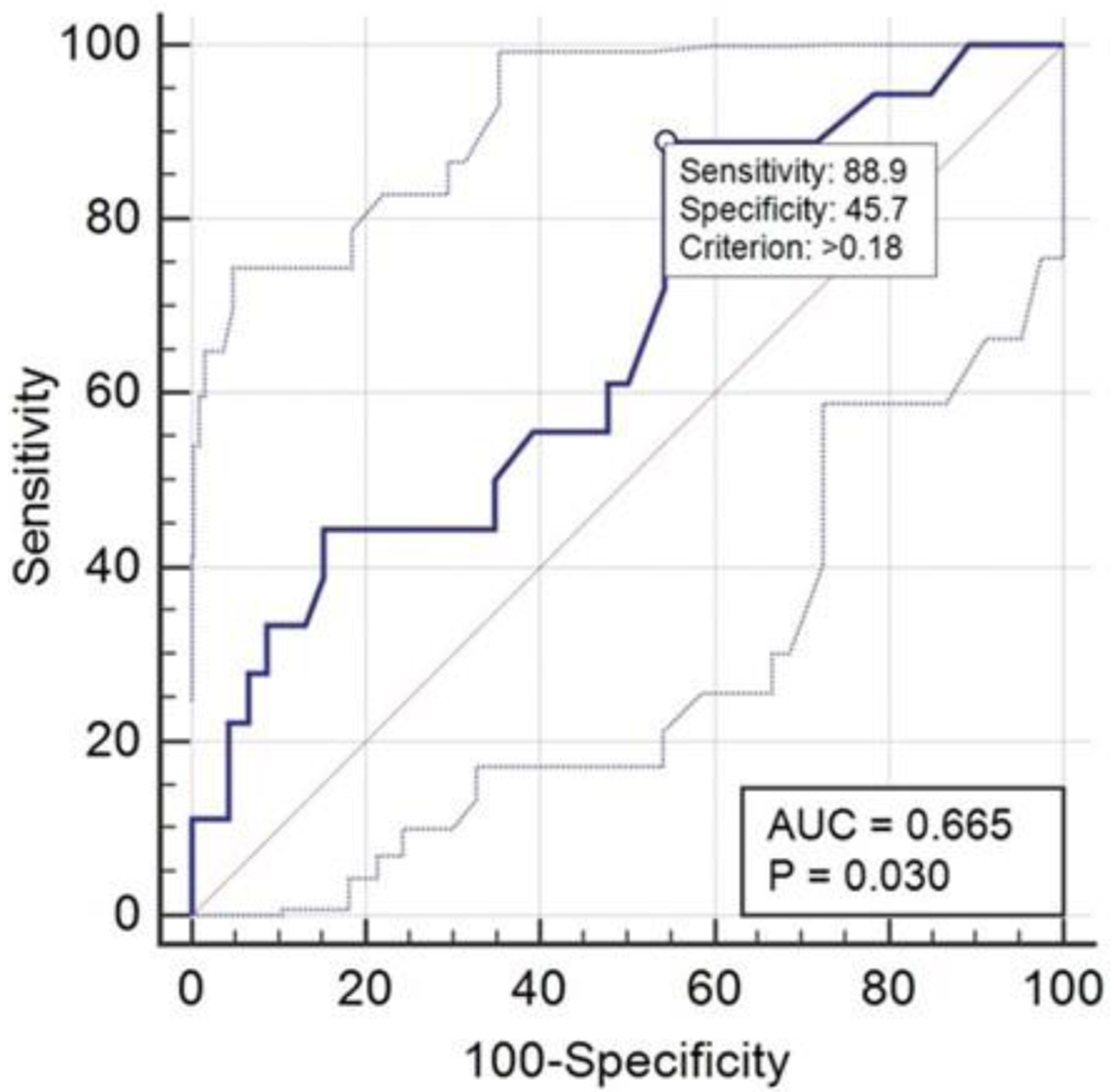 Click for large image | Figure 11. ROC curve relative to S100 performance. ROC: receiver operating characteristic. |
NFL dosage results
The results obtained from the NFL assay, conducted on 63 samples (Table 6), highlighted that 41 patients tested positive, despite the detected damage being of mild severity. Even the three patients with severe damage (no. 18, no. 19, and no. 114) and the two with moderate damage (no. 26 and no. 117) exhibited values higher than the threshold considered.
 Click to view | Table 6. Results Related to the NFL Marker Dosage |
The analysis of the NFL test performance for TBIs (Fig. 12) revealed a low accuracy, at 57%, and a low diagnostic specificity (44%). Despite these low percentages, the diagnostic sensitivity of the test is high (84%) and is accompanied by an NPV of 91% and a PPV of 39%.
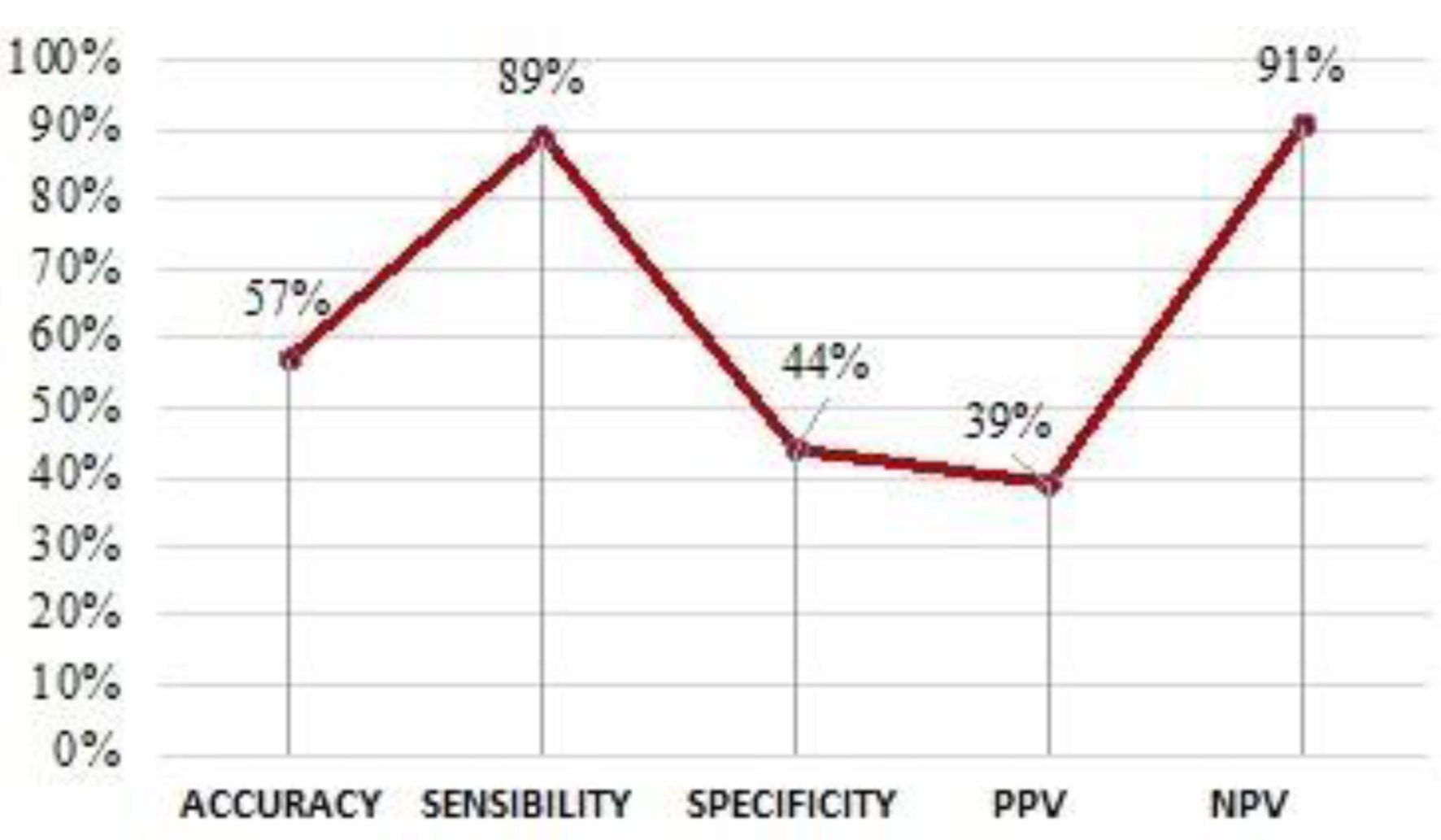 Click for large image | Figure 12. Scatter plot of NFL performance. NFL: neurofilament light chain. |
The analysis of the results using the ROC curve (Fig. 13), including the control group, confirmed a high sensitivity value (89%) and an increase in specificity from 44% to 57%.
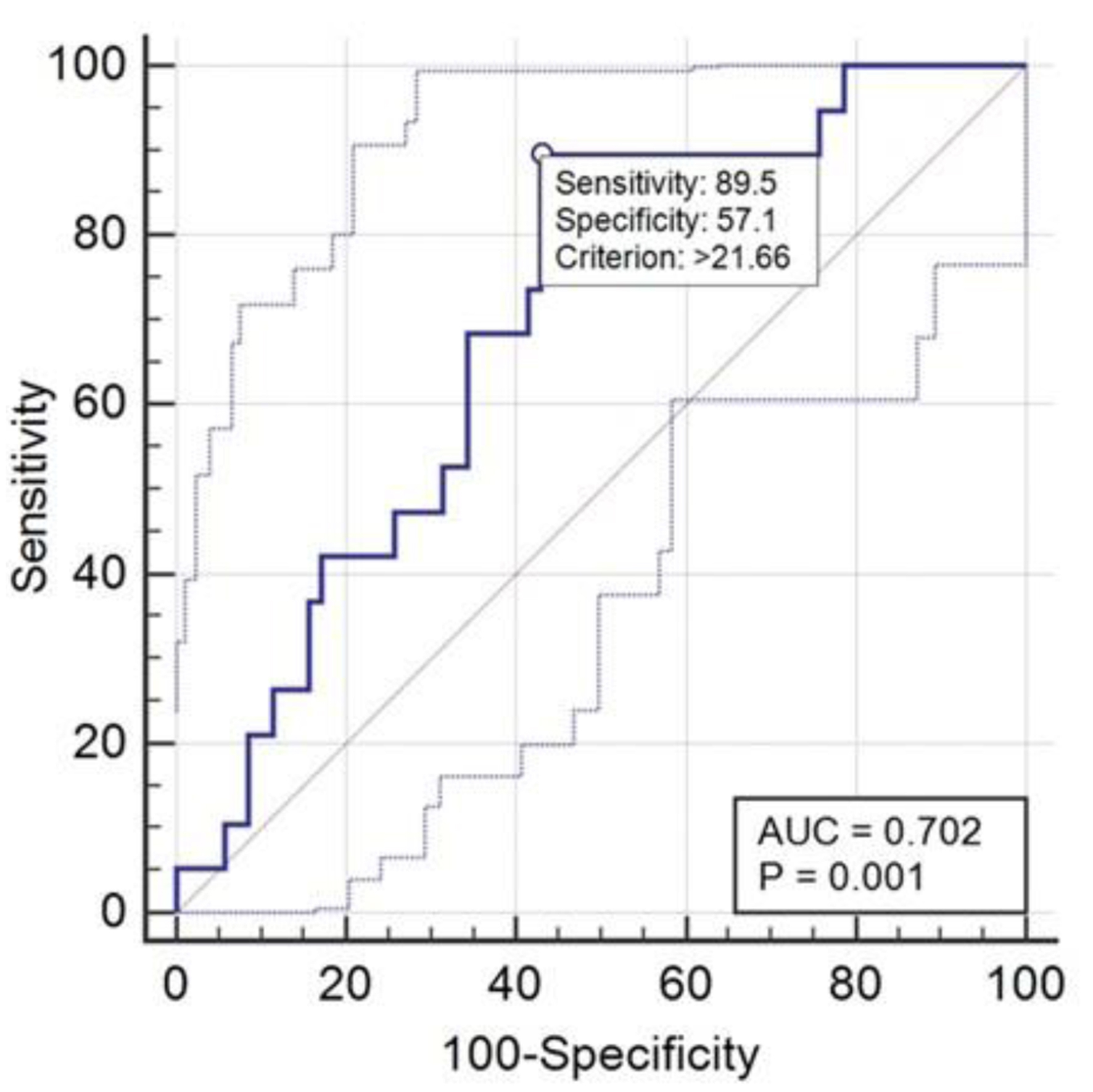 Click for large image | Figure 13. ROC curve relative to NFL performance. NFL: neurofilament light chain; ROC: receiver operating characteristic. |
Comparative analysis of performance regarding the five markers
The results regarding the comparison of the performance of the five markers dosed in this study as markers of cranial trauma have been summarized in Figure 14.
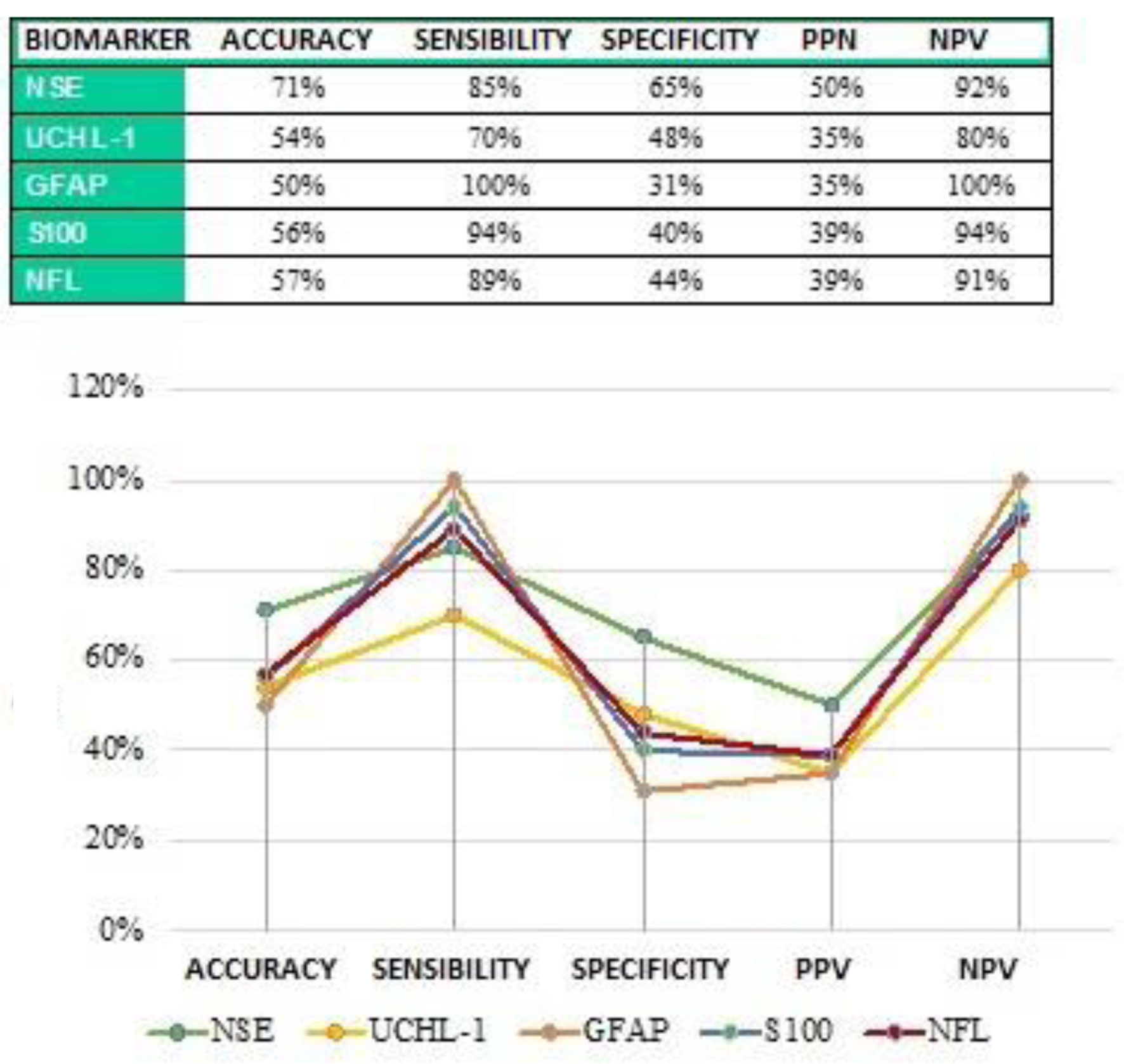 Click for large image | Figure 14. Comparison of the performance of the five dosed markers. |
The most evident data consist of the maximum percentage (100%) reached through the dosage of the GFAP marker in sensitivity and NPV values. This is also confirmed by the statistical comparison performed with overlaid ROC curves (Fig. 15).
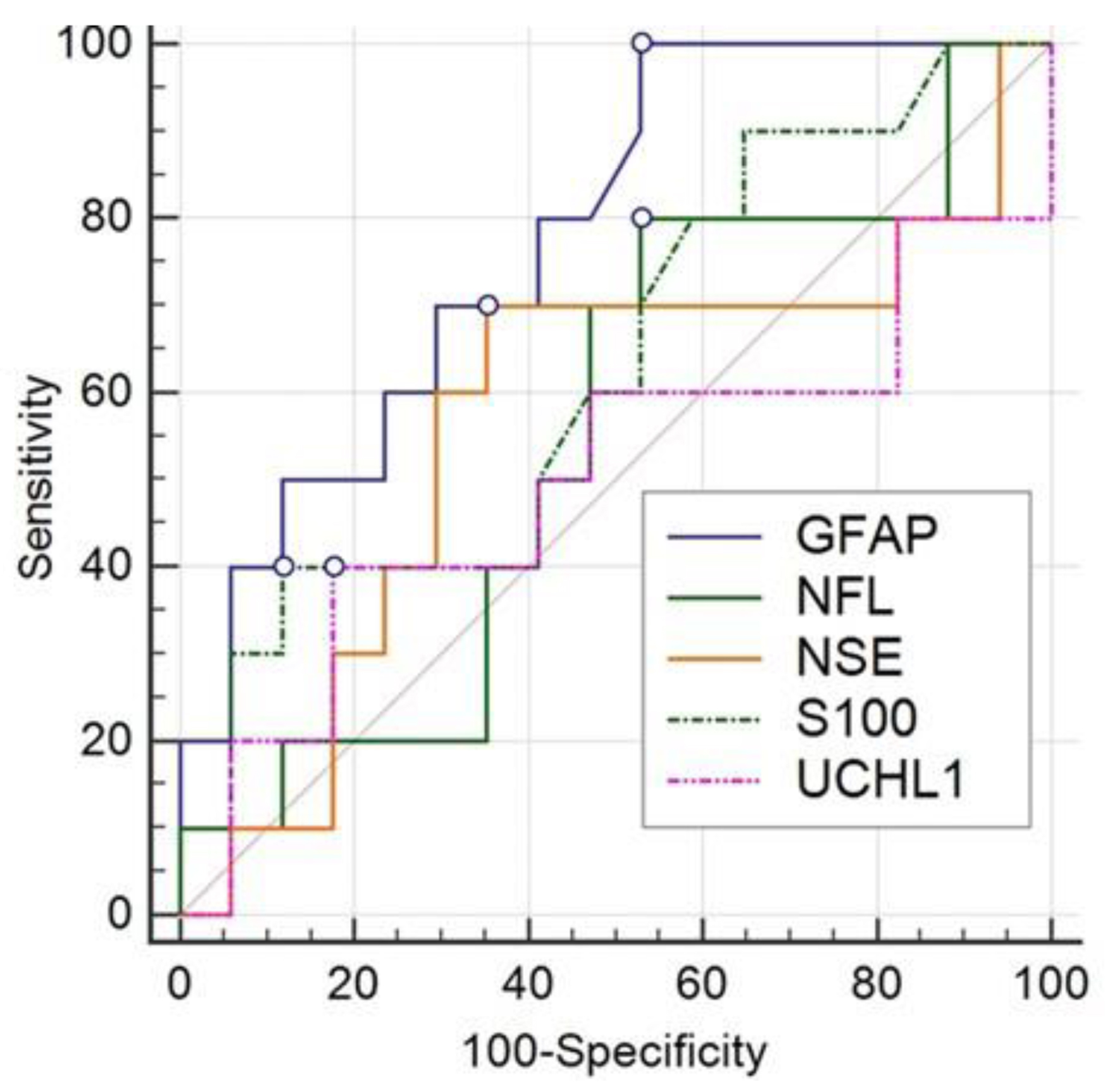 Click for large image | Figure 15. Comparison of ROC curves for the sensitivity attributed to the five dosed biomarkers. ROC: receiver operating characteristic. |
| Discussion and conclusions | ▴Top |
Over the past decade, significant scientific advancements have contributed to expanding the knowledge regarding the complex pathophysiological processes associated with TBI. Every year, approximately 60 million people experience cranial trauma of varying severity, classified as mild, moderate, or severe.
CT of the skull remains the main imaging modality for diagnosing intracranial lesions, such as hemorrhages or edema, in patients with cranial trauma treated in the emergency department during the acute post-traumatic period. CT, combined with patient symptoms and physical examinations, is crucial for guiding care for these patients.
However, this approach entails exposure to high doses of radiation and requires substantial healthcare resources and costs. Furthermore, this diagnostic technique can detect intracranial lesions in less than 10% of cases of mild to moderate TBI [16]. For these reasons, there has been a strong and growing interest in more objective clinical methodologies in identifying brain injuries, shifting attention to specific biomarkers, i.e., proteins present in the serum closely associated with TBI. This study focused on analyzing some TBI biomarkers, including NSE, S100B, NFL, UCH-L1, and GFAP, with the aim of conducting a preliminary assessment of patients with mild to moderate TBI.
NSE emerges as a highly specific marker for neurons and peripheral neuroendocrine cells [17]. However, it is important to highlight the multiple functions of this biomarker, as it is involved not only in the physiology of the central nervous system but also in other anatomical districts. Consistent with this evidence, in this study, NSE values were increased in all patients who, despite not showing acute cranial trauma damage on CT examination, presented signs of previous ischemic heart disease or stroke. Indeed, it is now known from the literature that NSE levels increase following brain tissue damage due to head injuries, as well as in response to ischemic stroke, intracerebral hemorrhage, inflammatory brain diseases, and Creutzfeldt-Jakob disease [17]. Furthermore, in laboratory diagnostics, NSE is mainly used in oncology: as a biomarker in neuroendocrine tumors and neuroblastoma [18], and, in combination with the gastrin-releasing peptide precursor (ProGRP), in monitoring small cell lung carcinoma (SCLC) [19]. Considering the low diagnostic sensitivity of the NSE test on TBI patients participating in this study and considering its wide range of applications in various areas of laboratory diagnostics, it can be concluded that NSE cannot be considered a useful biomarker for selecting emergency room patients with mild to moderate cranial trauma in relation to the need for CT examination.
S100, according to the literature, can be released from various body districts under multiple pathophysiological conditions, thus not being specific to nervous tissue. Based on the results obtained in this study, it emerges that the majority of patients with histories of heart disease or conditions related to metabolic syndrome showed a significant increase in S100 levels above reference values. An interesting note is that a significant percentage of these patients, despite the increase in S100, did not show intracranial lesions during the CT examination. These data confirm that S100 can also be released from adipose tissue and cardiac/skeletal muscles, as previously reported in the literature [12-14]. Therefore, an increase in the levels of this marker could occur even in the presence of lesions that do not involve the skull. Consequently, the results obtained in this study do not suggest using S100 as a biomarker associated with cranial trauma damage for predicting abnormalities in CT images and for assessing the development of post-concussion syndrome among patients with mild cranial trauma as supported by other studies [3].
Among the various biomarkers tested, there are also NFs (neurofilament light blood or NFL blood), whose diagnostic performances approach those of S100; unlike the latter, NFLs have the advantage of being exclusively expressed in neurons, constituting a fundamental structural element, playing an important role in axonal transport, and thus representing a potential biomarker indicative of axonal damage. In the general neurological context, this study confirms the primary role of NFLs whose levels, along with those of GFAP, are elevated in all five patients who report neurological or neurodegenerative pathologies in their medical history, four of whom have negative CT scans for cranial trauma lesions. Analyzing the results of NFLs dosed on the control group sera, as there are still few studies with consistent data on the healthy population, we relied on a study [20] that stratified age-specific reference values on a population of 1,724 healthy subjects aged 5 to 90 years using the “Simoa” method (Single Molecular Assay by Quanterix). Despite the use of a different NFL analysis method, the control group (average age 48 years) selected in this study had an average NFL value of 12.78 pg/mL, very close to the value of 10 pg/mL defined by the Simren study for healthy subjects aged 18 to 51 years.
The 95th percentile reference range was calculated in the range 9.98 - 15.58 pg/mL. In accordance with the Simren study, NFL values showed a correlation with increasing age. Only one NFL value was above the mean for its age, found in a subject with chronic ophthalmic headache. In this regard, it might be interesting to expand the diagnostic application of NFLs in a broader neurological context, beyond the roles already well defined in the literature, such as the association with diseases such as amyotrophic lateral sclerosis, Creutzfeldt-Jakob disease, and therapeutic and prognostic monitoring in multiple sclerosis [21]. Considering that, in this study, the TBI-involved patients had an average age of 70 years and that the median expression levels of NFLs in the healthy population for this age group from the Simren study were 20 pg/mL (from 61 to 70 years) and 35 pg/mL (> 70 years), with a detected clinical cutoff of 21 pg/mL, it is plausible that some false positives (negative CT and elevated NFLs) are linked to age-dependent NFL overexpression. Consequently, this contributes to reducing the sensitivity of the test.
The six patients aged between 61 and 70 years, with negative CT scans, showed an NFL average of 22 pg/mL, a value close to the 20 pg/mL cutoff established by the Simren study. Among the 27 patients over 70 years old (average age 81 years), with negative CT scans, only 15 of them (55%) showed NFL values below the 35 pg/mL cutoff proposed by Simren for healthy subjects in this age group. This difference is justified by the fact that the patients participating in the study reported in this study, although having negative CT scans for intracerebral traumatic lesions, cannot certainly be considered healthy. In fact, of the 12 patients with negative CT scans and NFL > 35 pg/mL, 10 of them (83%) had a clinical history or evidence of radiological imaging associated with previous episodes of ischemic or hemorrhagic strokes and heart diseases of various degrees. These data support the results obtained from recent studies, which confirm the clinical utility of NFLs in evaluating damage caused by different types of strokes and in predicting prognosis for affected patients [22].
The UCH-L1 test is provided by Abbott in conjunction with the GFAP test as a single diagnostic panel, called the TBI kit, to be used on the Alinity i analyzer. The diagnostic performances provided by the manufacturer refer to studies in which accuracy, specificity, and sensitivity are not separately indicated for the individual GFAP and UCH-L1 tests, but these parameters are provided cumulatively as overall sensitivity and specificity of the TBI kit [23]. The objective of this study was, therefore, to analyze the individual GFAP and UCH-L1 diagnostic kits separately since each biomarker refers to different cellular components and categories, as well as follows different kinetics and temporal profiles during the acute phase following the traumatic event. A first differentiation is related to patients with neurological or neurodegenerative pathologies, in which GFAP together with NFLs increased in all five cases, while UCH-L1 only in two of them. The UCH-L1 test has accuracy similar to the GFAP test but has slightly higher specificity.
The real substantial difference between the two tests is found in sensitivity. Indeed, while UCH-L1 has low sensitivity and low NPV, the GFAP test has been confirmed in this study as the best biomarker in terms of sensitivity and NPV as well. Based on the results obtained, as well as the low sensitivity, UCH-L1 cannot be recommended as a predictive marker of intracranial injury resulting from trauma in the context of this study. On the contrary, GFAP was the only test to achieve 100% sensitivity and 100% NPV. In practice, in all patients who, following trauma to CT, showed signs of intracranial lesions, GFAP was increased above the cutoff recommended by the manufacturer.
Based on the diagnostic performances observed on the five tests considered in this study, GFAP has emerged as a potential biomarker to be used in emergency medicine. Not showing any cases of false negatives, in fact, the diagnostic sensitivity of 100% of the GFAP test would allow, for serum values measured within 12 h of mild cranial trauma, lower than the cutoff of 35 pg/mL, to exclude with a good margin of safety patients to undergo cranial CT. This would mean a significant reduction in waiting times for emergency rooms and neuroradiology departments, a reduction in healthcare costs for instrumental investigations, ultimately avoiding unnecessary radiation exposure to the patient. Secondly, given the good correlation found with GFAP, the biomarkers that showed the best sensitivity and NPV results are NFLs and S100. However, NFL, compared to S100, has the advantage of having high tissue specificity, being expressed exclusively by nerve cells. In the subacute phase, beyond 12 h from the traumatic event, in addition to GFAP, the measurement of light chain neurofilaments could therefore also be useful, with a slower release into circulation compared to GFAP, with the aim of extending the patient monitoring time window related to the kinetics of molecule accumulation in the blood following cranial trauma. In case of mild trauma, with negative GFAP but positive NFLs, it would remain fundamental to resort to cranial CT examination to discriminate whether the elevation of NFs is associated with neuronal damage, vascular implications, or age-dependent biological variability. Initially, as an emergency medicine triage, it is not advisable to use NFLs due to limited sensitivity compared to GFAP. This limitation should be interpreted considering that NFLs, unlike GFAP, confirm, in accordance with recent literature, a progressive increase in expression levels in circulation with advancing age, and this could affect the sensitivity of the analysis when using the test for diagnostic purposes on people with mild cranial trauma whose average age is often quite advanced. In this regard, it would be appropriate in the future to expand the control group numerically in the study, including among healthy subjects with a rather advanced average age to define differentiated plasma NFs cutoffs for age groups.
Acknowledgments
None to declare.
Financial Disclosure
There was no financial support for this study.
Conflict of Interest
All other authors have no conflict of interest to disclose.
Informed Consent
Not applicable.
Author Contributions
Giambattista Lobreglio conceived and designed the study; structured the informed consent; contacted the interested companies for the supply of laboratory diagnostic kits; reviewed the results and the writing of the article. Giorgia Valentini participated in writing the manuscript and interfaced with the emergency room for the selection of cases of patients with head trauma. Marinella Marrazzi provided access to information and clinical and anamnestic news by approving the informed consent and submitting it to the emergency room doctors. Adriana Paladini provided access to the information contained in the reports of neuroradiology tests performed on patients with head trauma. Michele Chicone performed the serum dosages of the biomarkers, performing calibrations and quality controls on the laboratory diagnostic kits. He collected the data by processing the statistics and the results. He recruited the volunteer subjects who offered themselves as a healthy control group to establish the reference values of light chain neurofilaments and performed the blood sampling on them. He wrote most of the article.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
CT: computed tomography; GFAP: glial fibrillary acidic protein; GCS: Glasgow coma scale; mTBI: mild traumatic brain injury; NFL: neurofilament light chain; NSE: neuron-specific enolase; NPV: negative predictive value; PPV: positive predictive value; ROC: receiver operating characteristic; S100B: calcium-binding protein of astroglial origin; TBI: traumatic brain injury; UCH-L1: ubiquitin C-terminal hydrolase L1
| References | ▴Top |
- Matis G, Birbilis T. The Glasgow Coma Scale—a brief review. Past, present, future. Acta Neurol Belg. 2008;108(3):75-89.
pubmed - Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G. The Glasgow Coma Scale at 40 years: standing the test of time. Lancet Neurol. 2014;13(8):844-854.
doi pubmed - Wang KK, Yang Z, Zhu T, Shi Y, Rubenstein R, Tyndall JA, Manley GT. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev Mol Diagn. 2018;18(2):165-180.
doi pubmed - Mayer AR, Dodd AB, Dodd RJ, Stephenson DD, Ling JM, Mehos CJ, Patton DA, et al. Head kinematics, blood biomarkers, and histology in large animal models of traumatic brain injury and hemorrhagic shock. J Neurotrauma. 2023;40(19-20):2205-2216.
doi pubmed - Xu CM, Luo YL, Li S, Li ZX, Jiang L, Zhang GX, Owusu L, et al. Multifunctional neuron-specific enolase: its role in lung diseases. Biosci Rep. 2019;39(11):BSR20192732.
doi pubmed - Cunningham RT, Young IS, Winder J, O'Kane MJ, McKinstry S, Johnston CF, Dolan OM, et al. Serum neurone specific enolase (NSE) levels as an indicator of neuronal damage in patients with cerebral infarction. Eur J Clin Invest. 1991;21(5):497-500.
doi pubmed - Missler U, Wiesmann M, Friedrich C, Kaps M. S-100 protein and neuron-specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic stroke. Stroke. 1997;28(10):1956-1960.
doi pubmed - Shahjouei S, Sadeghi-Naini M, Yang Z, Kobeissy F, Rathore D, Shokraneh F, Blackburn S, et al. The diagnostic values of UCH-L1 in traumatic brain injury: A meta-analysis. Brain Inj. 2018;32(1):1-17.
doi pubmed - Jurga AM, Paleczna M, Kadluczka J, Kuter KZ. Beyond the GFAP-astrocyte protein markers in the brain. Biomolecules. 2021;11(9):1361.
doi pubmed - Rezaei O, Pakdaman H, Gharehgozli K, Simani L, Vahedian-Azimi A, Asaadi S, Sahraei Z, et al. S100 B: A new concept in neurocritical care. Iran J Neurol. 2017;16(2):83-89.
pubmed - Bersani I, Pluchinotta F, Dotta A, Savarese I, Campi F, Auriti C, Chuklantseva N, et al. Early predictors of perinatal brain damage: the role of neurobiomarkers. Clin Chem Lab Med. 2020;58(4):471-486.
doi pubmed - Goncalves CA, Leite MC, Guerra MC. Adipocytes as an important source of serum S100B and possible roles of this protein in adipose tissue. Cardiovasc Psychiatry Neurol. 2010;2010:790431.
doi pubmed - Parker TG, Marks A, Tsoporis JN. Induction of S100b in myocardium: an intrinsic inhibitor of cardiac hypertrophy. Can J Appl Physiol. 1998;23(4):377-389.
doi pubmed - Riuzzi F, Sorci G, Arcuri C, Giambanco I, Bellezza I, Minelli A, Donato R. Cellular and molecular mechanisms of sarcopenia: the S100B perspective. J Cachexia Sarcopenia Muscle. 2018;9(7):1255-1268.
doi pubmed - Coppens S, Lehmann S, Hopley C, Hirtz C. Neurofilament-light, a promising biomarker: analytical, metrological and clinical challenges. Int J Mol Sci. 2023;24(14):11624.
doi pubmed - Bazarian JJ, Welch RD, Caudle K, Jeffrey CA, Chen JY, Chandran R, McCaw T, et al. Accuracy of a rapid glial fibrillary acidic protein/ubiquitin carboxyl-terminal hydrolase L1 test for the prediction of intracranial injuries on head computed tomography after mild traumatic brain injury. Acad Emerg Med. 2021;28(11):1308-1317.
doi pubmed - Isgro MA, Bottoni P, Scatena R. Neuron-specific enolase as a biomarker: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:125-143.
doi pubmed - Mercier E, Tardif PA, Cameron PA, Emond M, Moore L, Mitra B, Ouellet MC, et al. Prognostic value of neuron-specific enolase (NSE) for prediction of post-concussion symptoms following a mild traumatic brain injury: a systematic review. Brain Inj. 2018;32(1):29-40.
doi pubmed - Molina R, Marrades RM, Auge JM, Escudero JM, Vinolas N, Reguart N, Ramirez J, et al. Assessment of a combined panel of six serum tumor markers for lung cancer. Am J Respir Crit Care Med. 2016;193(4):427-437.
doi pubmed - Simren J, Andreasson U, Gobom J, Suarez Calvet M, Borroni B, Gillberg C, Nyberg L, et al. Establishment of reference values for plasma neurofilament light based on healthy individuals aged 5-90 years. Brain Commun. 2022;4(4):fcac174.
doi pubmed - Benkert P, Meier S, Schaedelin S, Manouchehrinia A, Yaldizli O, Maceski A, Oechtering J, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol. 2022;21(3):246-257.
doi pubmed - Gendron TF, Badi MK, Heckman MG, Jansen-West KR, Vilanilam GK, Johnson PW, Burch AR, et al. Plasma neurofilament light predicts mortality in patients with stroke. Sci Transl Med. 2020;12(569):eaay1913.
doi pubmed - Bazarian JJ, Biberthaler P, Welch RD, Lewis LM, Barzo P, Bogner-Flatz V, Gunnar Brolinson P, et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): a multicentre observational study. Lancet Neurol. 2018;17(9):782-789.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.