| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website https://jnr.elmerpub.com |
Original Article
Volume 15, Number 2, March 2025, pages 71-76
The Impact of Metabolic Control on Limb Differences in Peripheral Neuropathy: A Pilot Study
Abigayle B. Simona, Charles J. Weeksa, Ryan A. Harrisa, c, Sudhir S. Athnib
aMedical College of Georgia, Augusta University, Augusta, GA, USA
bNeurology of Central Georgia, Macon, GA, USA
cCorresponding Author: Ryan A. Harris, Medical College of Georgia, Augusta University, Augusta, GA 30912, USA
Manuscript submitted November 5, 2024, accepted January 18, 2025, published online January 25, 2025
Short title: Metabolic Control and Limb Differences in Peripheral Neuropathy
doi: https://doi.org/10.14740/jnr865
| Abstract | ▴Top |
Background: Hyperglycemia is thought to be one of the most common causes of peripheral neuropathy. Pathology of the peripheral nervous system can involve sensory nerves, motor nerves, or autonomic nerves. It is typically believed that hyperglycemia-induced neuropathies follow a length-dependent pattern, generally affecting the feet and toes first, with the hands and fingers involved at a later stage. Sudomotor function, which is the body’s ability to regulate sweat production through the autonomic nervous system, is often one of the earliest signs of autonomic dysfunction. There is limited information on the inter-limb differences in peripheral neuropathy, and the precise impact of metabolic control on individual limbs has yet to be clarified. Thus, the present study aims to explore the relationships between glucose regulation and peripheral neuropathy across all four limbs, using sudomotor activity as a measure of nerve functional status.
Methods: Electronic medical record data from 34 individuals of a private practice clinic were included in this study. Fasting blood glucose and hemoglobin A1c (HbA1c) were used as indices of metabolic control. Sudomotor function was assessed using the Sudoscan device and serves as an important indicator of peripheral autonomic nerve function. Sudomotor function is measured in electrochemical skin conductance (ESC), with a lower ESC representing greater peripheral neuropathy.
Results: There were no differences in patient demographics or clinical laboratory values between men and women (all P > 0.05), except that men were taller (P < 0.001) and heavier (P = 0.021) compared to women. The right hand had a lower ESC than the left hand (P < 0.001), left foot (P < 0.001), and right foot (P < 0.001). The left hand had a lower ESC than the left foot (P = 0.005) and the right foot (P = 0.003). There was a significant negative relationship between fasting blood glucose concentrations and ESC in the left hand (r = -0.363; P = 0.035), right hand (r = -0.474; P = 0.005), and right foot (r = -0.385; P = 0.024).
Conclusion: Contrary to popular belief, the results of the present investigation suggest that hands have more severe peripheral autonomic neuropathy compared to the feet. Lastly, there is a direct linear relationship between degree of peripheral autonomic neuropathy and fasting blood glucose and age. Indeed, current findings suggest a potential need for a shift in clinical practice, where more detailed testing of the hands and arms could detect early signs of neuropathy before it becomes more clinically evident in the legs.
Keywords: Hyperglycemia; Sudomotor function; Diabetes; Sudoscan
| Introduction | ▴Top |
Peripheral neuropathy is a term that is used to describe any pathology causing damage to the nerves in the peripheral nervous system, with the exact pathophysiology of this condition contingent on the underlying disease [1]. Close to 2.4% of the world population is affected by peripheral nerve disorders, and this number increases to 8% in older individuals [2]. The most common cause of peripheral neuropathy is hyperglycemia, which can contribute to end-organ damage long before a formal diagnosis of diabetes mellitus is confirmed [3]. Sustained hyperglycemia over time, resulting in an increased hemoglobin A1c (HbA1c), has multiple adverse effects on the body, including damaging the small penetrating blood vessels that supply nerves with nutrition and oxygen needed for normal function. Such chronic hyperglycemia also contributes to insulin resistance resulting in oxidative stress, inflammation, and overall cell damage [4]. Approximately 50% of individuals with hyperglycemia will experience peripheral neuropathy at some point in their lives [5], and elevated HbA1c has been shown to correlate with a more severe peripheral neuropathy [6]. Indeed, peripheral neuropathy is an independent risk factor for the development of cardiovascular disease (CVD) [7]. Alternatively, various cardiovascular risk factors including hyperlipidemia and hypertriglyceridemia themselves are independent risk factors for the development of peripheral neuropathy [8].
Hyperglycemia-associated peripheral neuropathy can affect the sensory, motor, and autonomic nerves and typically starts in the extremities. Following a nerve-length-dependent pattern, the feet and toes are impacted first, whereas neuropathy in the hands and fingers is typically identified later [9]. Specifically, sudomotor function, the body’s ability to regulate sweat production through the autonomic nervous system, is the first to be affected in autonomic neuropathy [10]. Indeed, the degree of sudomotor dysfunction is associated with severity of peripheral neuropathy [11]. Despite autonomic neuropathy often occurring first, patients rarely come to the office with concerns regarding reduced sweating or other subtle signs of autonomic dysfunction. Sensory changes, like numbness or tingling, tend to be more noticeable and concerning for patients, which is why they are usually the primary reason for consultation, even though autonomic issues may already be present. Thus, it is conceivable that early detection of sudomotor dysfunction could reduce CVD risk and overt disease and events. Multiple studies have validated the use of sudomotor function assessment in clinical practice and in individuals with type 2 diabetes [12-14]. However, there remain limited data on limb-specific differences and the relationship between glycemic control and these inter-limb variations. Specifically, it remains unclear whether the severity of neuropathy is consistent between the upper and lower limbs. While there is limited information on inter-limb differences in peripheral neuropathy, the precise impact of metabolic control on peripheral neuropathy in individual limbs has yet to be elucidated. Understanding the differential impact of peripheral neuropathy on various limbs could provide further insights into the early detection, pathophysiology, management and treatment of this condition. Therefore, the purpose of the present study is to explore the relationships between glucose regulation and peripheral neuropathy within all four limbs of the body. It was hypothesized that the lower and left limbs would have greater degrees of sudomotor dysfunction than the upper and right limbs.
| Materials and Methods | ▴Top |
Study population and data collection
A total of 34 individuals (men: n = 8; women: n = 26) were included in this retrospective chart review. Patients included in the study were those who received Sudoscan testing during office visits between November 2023 and February 2024. This screening was done to differentiate between a peripheral or central etiology of neurologic symptoms in patients without previous diagnostic testing. Participants were excluded if they did not have clinical laboratory values drawn within 3 months of testing. All participants reported to the Neurology of Central Georgia office having abstained from moderate-vigorous exercise for 12 h prior to testing. Patient confidentiality was maintained throughout the study and utilized only pre-existing, de-identified data. Accordingly, the research was considered non-human subjects research per the Augusta University IRB office.
Indices of metabolic control
Fasting blood glucose and HbA1c were used as indices of metabolic control. Blood urea nitrogen (BUN) was used as an index of renal function. Vitamins B12, folate, and vitamin D were assessed in available participants to rule out vitamin deficiency as a cause of peripheral neuropathy.
Peripheral neuropathy
Sudomotor function, the body’s ability to regulate sweat production through the autonomic nervous system, was assessed using the Sudoscan device (Sudoscan, San Diego, CA), and serves as an indicator of peripheral nerve function. This test is a new, rapid method based on the electrochemical interaction between sweat chloride and stainless-steel electrodes. This approach is valid and reproducible in both in vitro and clinical studies [15-17]. To minimize any possible interference and to obtain a clean sudomotor function assessment, patients were instructed to avoid applying any ointments or lotions to their body. During the test, patients laid in a supine position, placed their hands and feet on stainless-steel electrodes, and a low, incremental direct voltage (< 4 V) was applied for approximately 2 min. Electrochemical skin conductance (ESC), a marker of sudomotor function, was calculated as the ratio of the measured current to the applied voltage. The results of the test are expressed as ESC (microsiemens, µS) for both hands and feet. An ESC between 0 and 40 µS is indicative of severely reduced sudomotor function, between 40 and 60 µS is indicative of moderately reduced sudomotor function, and between 60 and 100 µS is indicative of normal sudomotor function.
Statistical analysis
Statistical analyses were performed using SPSS Version 29. All data are expressed as mean ± standard error of mean (SEM) unless otherwise noted. For differences in demographic and clinical laboratory variables, Chi-square tests or independent samples t-tests were used. For sex differences, independent sample t-tests were used. A repeated measures analysis of variance (ANOVA) was used to determine inter-limb differences. A Pearson correlation was used to determine relationships among the degree of peripheral neuropathy, metabolic control, and age. Post hoc pairwise comparisons were performed using a Bonferroni adjustment to the overall alpha level for the number of comparisons. Effect sizes for differences in peripheral neuropathy are reported as Cohen’s d values to represent small (Cohen’s d = 0.2), medium (Cohen’s d = 0.5), and large (Cohen’s d = 0.8) effect sizes (26). Statistical significance was set at P < 0.05.
| Results | ▴Top |
Patient demographics and clinical laboratory values are presented in Table 1. Men were taller (P < 0.001) and heaver (P = 0.021) compared to women. There were no other differences observed (all P > 0.05). There was no limb-sex interaction observed (all P > 0.05). No sex differences were observed in any limb ESC between men and women (all P > 0.05).
 Click to view | Table 1. Overall Participant Characteristics |
Figure 1 illustrates the ESC variations across different limbs. The right hand had a lower ESC than the left hand (Cohen’s d = -0.834; P < 0.001), left foot (Cohen’s d = -0.819; P < 0.001), and right foot (Cohen’s d = -0.875; P < 0.001). The left hand had a lower ESC than the left foot (Cohen’s d = -0.510; P = 0.005) and right foot (Cohen’s d = -0.543; P = 0.003). There were no differences in ESC between the left and right foot (P > 0.05).
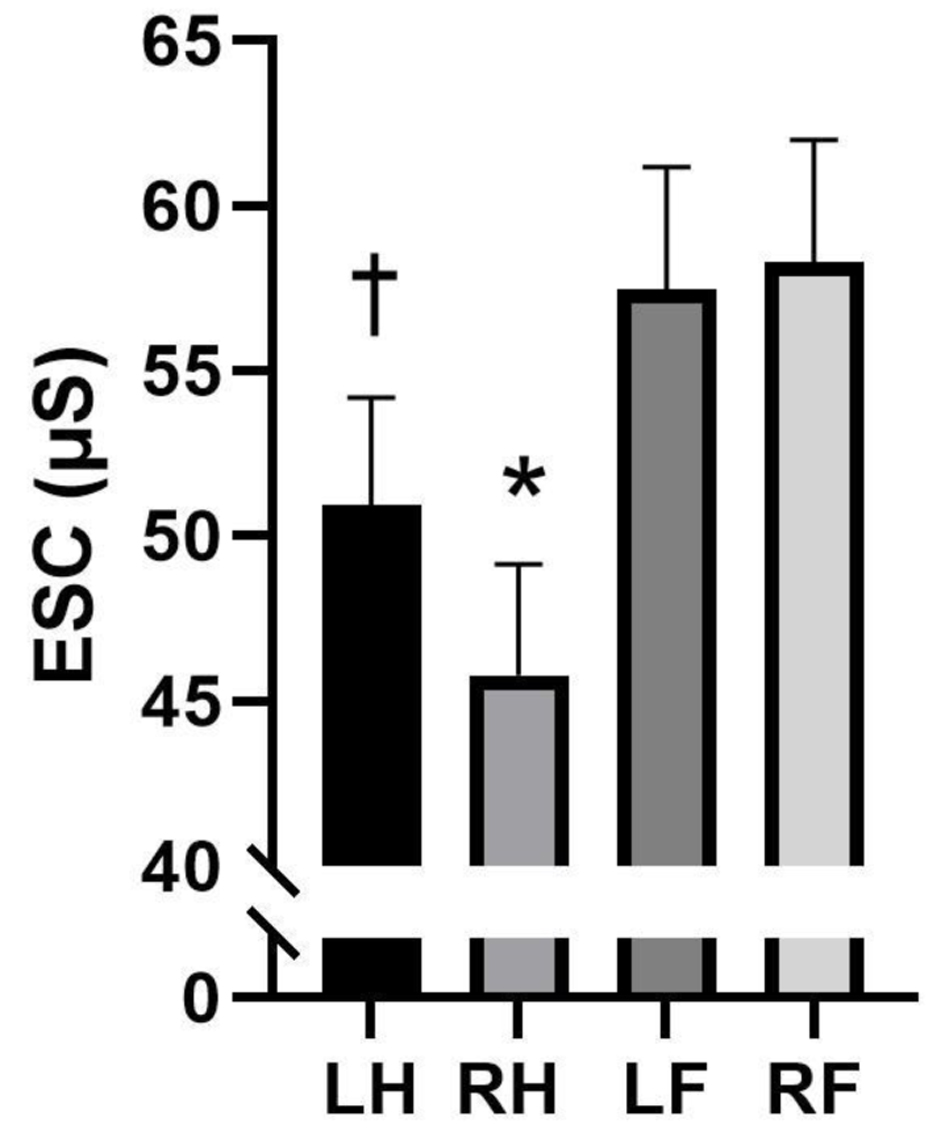 Click for large image | Figure 1. Differences in electrochemical skin conductance (ESC) differences between limbs (n = 34). Analysis of variance. Data are presented as mean ± standard error of mean (SEM). *Statistically significant from left hand (LH), left foot (LF), and right foot (RF). †Statistically significant from LF and RF. |
The relationships between fasting glucose and ESC across different limbs are shown in Figure 2. There was a significant negative relationship between fasting blood glucose concentrations and ESC in the left hand (r = -0.363; P = 0.035) (Fig. 2a) right hand (r = -0.474; P = 0.005) (Fig. 2b), and right foot (r = -0.385; P = 0.024) (Fig. 2c). This relationship was not seen with the left foot (r = -0.274; P = 0.117). There was no relationship between HbA1c and ESC in any extremity. In addition, there was a significant negative relationship between age and ESC in the left hand (r = -0.515; P = 0.002), right hand (r = -0.486; P = 0.004), left foot (r = 0.520; P = 0.002), and right foot (r = -0.533; P = 0.001). Further, there was a significant positive relationship between age and fasting glucose (r = 0.343; P = 0.047).
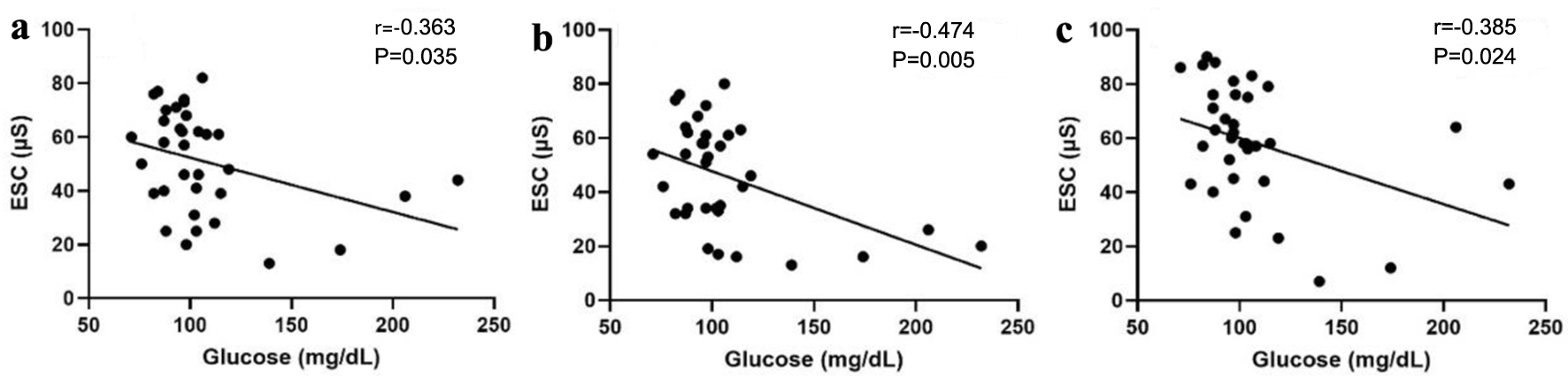 Click for large image | Figure 2. Relationships between fasting glucose and electrochemical skin conductance (ESC) in (a) left hand (LH), (b) right hand (RH), and (c) right foot (RF) (n = 34). Pearson’s correlation. |
| Discussion | ▴Top |
Peripheral neuropathy affects millions of people worldwide, and its prevalence is closely linked to poor glycemic control, particularly in individuals with diabetes. Although it has been shown that peripheral neuropathy starts in the lower extremities, comparing the degree of neuropathy in the individual extremities has not yet been studied. The results from the present study demonstrate that hands have a greater degree of peripheral autonomic neuropathy when compared to the feet. Additionally, there is a positive linear relationship between the degree of autonomic neuropathy and both fasting glucose and age. This finding challenges the common assumption that neuropathy progression always follows a distal-to-proximal pattern, suggesting that a more complex mechanism may be at play.
Extremity difference in peripheral neuropathy
Peripheral neuropathy caused by hyperglycemia is often assumed to follow a length-dependent pattern [9], suggesting that the legs, being longer, would show signs of damage before the arms. However, the current study presents an interesting paradox, as the arms appear to be more severely affected than the legs. It is important to note that despite the small sample size in this study, the Cohen’s d values indicate a large effect size. Nonetheless, the present findings raise the question of whether the arms could be more susceptible to hyperglycemia-induced damage earlier than the legs. While damage still progresses in a distal-to-proximal manner, it is possible that the arms are initially impacted by hyperglycemia before the legs. Despite this possibility, most neurologists primarily focus on testing the legs and feet during clinical exams, under the assumption that they are affected first. The arms, by contrast, are typically examined only when the condition is severe and reported by the patient. Present findings indicate the need for a paradigm shift in how neuropathy is diagnosed and monitored in a clinical setting. By conducting more detailed tests on the hands and arms early on, it may be possible to catch signs of neuropathy that would otherwise go undetected in its initial stages. Objective testing using the Sudoscan, which can detect sudomotor dysfunction, might allow for earlier detection of neuropathy in the arms before it manifests in the legs, an observation that will provide indication for early diagnosis, treatment, and management. Indeed, future research with a larger patient cohort could certainly further validate these findings.
The current study does not examine the timing and onset of neuropathy. Instead, the results suggest that, irrespective of when neuropathy begins, both hands exhibit a greater degree of peripheral neuropathy compared to both feet. Notably, the right hand, on average, shows a higher degree of peripheral neuropathy compared to the left hand. While the exact mechanism behind this observation remains unclear, handedness may be a contributing factor. Although the hand dominance of the participants in the current study is unknown, 90% of the population is right-handed [18]. It is hypothesized that the increased use of the right hand, which is dominant in most individuals, contributes to a higher degree of neuropathy in that hand. However, it could also be argued that frequent use of the dominant hand might actually preserve nerve function, thereby offering some protection against neuropathy. This dual perspective raises questions about the relationship between nerve use and degradation. Future research should explore how repetitive movements or tasks impact neuropathy progression in dominant versus non-dominant limbs and investigate whether neural protection mechanisms are at play in heavily utilized extremities. Understanding these dynamics could inform more personalized approaches to managing peripheral neuropathy. Recent literature indicates that left-handed individuals exhibit a greater CVD risk compared to right-handed individuals [19]. Given that peripheral neuropathy is an independent predictor of CVD risk and represents a form of microvascular dysfunction [7], findings of the present investigation provide support that repeated asymmetric hand usage may disproportionately accelerate the degree of peripheral neuropathy in the dominant hand, potentially increasing future CVD risk. Future studies are certainly needed to elaborate the role of handedness on peripheral neuropathy and test this proposed hypothesis.
Neuropathy is a significant form of end-organ damage often associated with chronic conditions like diabetes. Detecting neuropathy at an early stage could serve as an indicator of the onset of end-organ damage, allowing for timely intervention. Early identification of neuropathy could prompt more rigorous screening for other complications of hyperglycemia, such as renal dysfunction, retinopathy, and cardiovascular involvement, enabling healthcare providers to manage these issues proactively. By initiating preventive care sooner, morbidity and mortality related to these conditions could potentially be reduced. Additionally, implementing an inexpensive diagnostic test for early neuropathy detection could yield substantial cost savings for the healthcare system, potentially amounting to billions of dollars, by minimizing the need for more intensive treatments and hospitalizations later on.
Relationship of metabolic control and age on peripheral neuropathy
Diabetes is the leading cause of peripheral neuropathy and poorly controlled diabetes has been associated with higher rates of painful peripheral neuropathy [20]. The results of the current study suggest that higher fasting glucose is associated with a higher degree of peripheral neuropathy, regardless of extremity. Interestingly, no relationships between HbA1c and degree of peripheral neuropathy in any extremity were observed. However, there were missing HbA1c data in half of the participants in this study, so their data were not used in the analysis. Indeed, circulating blood glucose can fluctuate throughout the day and may influence the severity of peripheral neuropathy. While this study focused solely on fasting blood glucose, future research that utilizes continuous glucose monitoring to determine the relationship between glucose over time and sudomotor function is certainly warranted. Such an approach would provide a more comprehensive and precise assessment of the relationship between glycemic variability and peripheral neuropathy.
Age is a well-established risk factor for both peripheral neuropathy and CVD, with the prevalence of these conditions increasing significantly as individuals grow older. The aging process contributes to cumulative damage in both the peripheral nervous and cardiovascular systems, amplifying the risk of developing these disorders. The majority of the individuals in this study are relatively healthy individuals. The data have a broad range in terms of age (range: 21 - 80 years) and fasting glucose (range: 71 - 232 mg/dL), which gives a good representation on the general population. Age certainly plays a role in the progression of peripheral neuropathy regardless of glucose status, which is shown by the present findings. However, it is important to note that a positive relationship (all P < 0.05) remains with degree of peripheral neuropathy and age, when controlling for glucose, in all four extremities. This suggests that as individuals age, the severity of neuropathy increases, regardless of their glucose control, in all limbs. Elevated glucose levels can begin to cause nerve damage, but not all individuals receive an official diagnosis of peripheral neuropathy. Ultimately, assessing sudomotor function is as a way of measuring the degree and monitoring progression of peripheral neuropathy. Early detection using a non-invasive test may improve the specificity of diagnosing peripheral neuropathy, enabling timely intervention to manage both hyperglycemia and the neuropathy itself.
Conclusions
Neuropathy, by definition, encompasses sensory, motor, and autonomic components, though patients predominantly report sensory symptoms such as numbness, tingling, or pain. It is rare for patients to directly mention autonomic dysfunction, as symptoms like altered sweating patterns often go unnoticed. However, it is important to highlight that the Sudoscan specifically evaluates autonomic function, particularly sweat gland activity, which can be affected early in the course of neuropathy. Our findings suggest that autonomic dysfunction can present earlier in the arms than in the legs, offering a unique insight into the progression of the disease. The results of the present investigation suggest that hands have more severe peripheral neuropathy compared to feet. Lastly, there is a direct linear relationship between degree of peripheral neuropathy and fasting blood glucose and age. Future studies are needed to provide mechanistic insight into the inter-limb differences of peripheral neuropathy and the implications of these inter-limb differences. Indeed, current findings suggest a potential need for a shift in clinical practice, where more detailed testing of the hands and arms could detect early signs of neuropathy before it progresses to the legs. Non-invasive and easily accessible methods of sudomotor function may enable earlier diagnosis, allowing for better management of hyperglycemia-induced peripheral neuropathy.
Acknowledgments
The authors would like to thank the participants for their time.
Financial Disclosure
No funding was used in the conception of this manuscript.
Conflict of Interest
The authors have no conflict of interest to declare.
Informed Consent
Patient confidentiality was maintained throughout the study and utilized only pre-existing, de-identified data, thus exempting the need for informed consent. Accordingly, the research was considered non-human subjects research per the Augusta University IRB office.
Author Contributions
ABS analyzed data, interpreted results of experiments, prepared figures, drafted manuscript, edited and revised manuscript, and approved final version of manuscript. CJW interpreted results of experiments, edited and revised manuscript, and approved final version of manuscript. RAH interpreted results of experiments, edited and revised manuscript, and approved final version of manuscript. SSA conceived and designed research, analyzed data, interpreted results of experiments, drafted manuscript, edited and revised manuscript, and approved final version of manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Hanewinckel R, Ikram MA, Van Doorn PA. Peripheral neuropathies. Handb Clin Neurol. 2016;138:263-282.
doi pubmed - Hughes RA. Peripheral neuropathy. BMJ. 2002;324(7335):466-469.
doi pubmed - Amelia R, Wahyuni AS, Yunanda Y. Diabetic neuropathy among type 2 diabetes mellitus patients at amplas primary health care in medan city. Open Access Maced J Med Sci. 2019;7(20):3400-3403.
doi pubmed - Bodman MA, Dreyer MA, Varacallo MA. Diabetic peripheral neuropathy. In: StatPearls. Treasure Island (FL) ineligible companies. 2025.
pubmed - Hicks CW, Selvin E. Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr Diab Rep. 2019;19(10):86.
doi pubmed - Nozawa K, Ikeda M, Kikuchi S. Association between HbA1c levels and diabetic peripheral neuropathy: a case-control study of patients with type 2 diabetes using claims data. drugs real world outcomes. 2022;9(3):403-414.
doi pubmed - AlGhamdi G, Saati H, Almotairi E, Alsofiani BS, Kinsara AJ. Peripheral neuropathy as a risk factor for developing cardiovascular disease in diabetic patients. Cureus. 2020;12(12):e11922.
doi pubmed - Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes. 2009;58(7):1634-1640.
doi pubmed - Misra UK, Kalita J, Nair PP. Diagnostic approach to peripheral neuropathy. Ann Indian Acad Neurol. 2008;11(2):89-97.
doi pubmed - Akbar M, Wandy A, Soraya GV, Goysal Y, Lotisna M, Basri MI. Sudomotor dysfunction in diabetic peripheral neuropathy (DPN) and its testing modalities: A literature review. Heliyon. 2023;9(7):e18184.
doi pubmed - Carbajal-Ramirez A, Hernandez-Dominguez JA, Molina-Ayala MA, Rojas-Uribe MM, Chavez-Negrete A. Early identification of peripheral neuropathy based on sudomotor dysfunction in Mexican patients with type 2 diabetes. BMC Neurol. 2019;19(1):109.
doi pubmed - Chiu LT, Lin YL, Wang CH, Hwu CM, Liou HH, Hsu BG. Electrochemical Skin Conductance by Sudoscan in Non-Dialysis Chronic Kidney Disease Patients. J Clin Med. 2023;13(1):187.
doi pubmed - Lin K, Wu Y, Liu S, Huang J, Chen G, Zeng Q. The application of sudoscan for screening microvascular complications in patients with type 2 diabetes. PeerJ. 2022;10:e13089.
doi pubmed - Hussein, II, Alshammary SHA, Al-Nimer MSM. Assessment of sudomotor function in hypertensive with/without type-2 diabetes patients using SUDOSCAN: An electrophysiological study. Clin Neurophysiol Pract. 2021;6:22-28.
doi pubmed - Casellini CM, Parson HK, Richardson MS, Nevoret ML, Vinik AI. Sudoscan, a noninvasive tool for detecting diabetic small fiber neuropathy and autonomic dysfunction. Diabetes Technol Ther. 2013;15(11):948-953.
doi pubmed - Gin H, Baudoin R, Raffaitin CH, Rigalleau V, Gonzalez C. Non-invasive and quantitative assessment of sudomotor function for peripheral diabetic neuropathy evaluation. Diabetes Metab. 2011;37(6):527-532.
doi pubmed - Gavan DE, Gavan A, Bondor CI, Florea B, Bowling FL, Inceu GV, Colobatiu L. SUDOSCAN, an innovative, simple and non-invasive medical device for assessing sudomotor function. Sensors (Basel). 2022;22(19):7571.
doi pubmed - Coren S, Porac C. Fifty centuries of right-handedness: the historical record. Science. 1977;198(4317):631-632.
doi pubmed - Simon AB, Norland K, Blackburn M, Zhao S, Wang X, Harris RA. Evidence of increased cardiovascular disease risk in left-handed individuals. Front Cardiovasc Med. 2023;10:1326686.
doi pubmed - Pop-Busui R, Ang L, Boulton AJM, Feldman EL, Marcus RL, Mizokami-Stout K, Singleton JR, et al. Diagnosis and treatment of painful diabetic peripheral neuropathy. Arlington (VA). 2022;2022(1):1-32.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.