| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website https://jnr.elmerpub.com |
Case Report
Volume 000, Number 000, July 2025, pages 000-000
Rapidly Progressive Multifocal Cerebral Vasculitis Presenting With Bi-Hemispheric Stroke
Yiorgos Antoniadisa, Taseal Ahmeda, d, Chandrika Sanapalab, Samir Ruxmohanc
aSt. George’s University School of Medicine, True Blue, Grenada
bBurrell College of Osteopathic Medicine, Las Cruces, NM 88001, USA
cExceed Healthcare, Dallas, TX 75208, USA
dCorresponding Author: Taseal Ahmed, St. George’s University School of Medicine, True Blue, Grenada
Manuscript submitted May 9, 2025, accepted June 28, 2025, published online July 8, 2025
Short title: Cerebral Vasculitis With Bilateral Stroke
doi: https://doi.org/10.14740/jnr1034
| Abstract | ▴Top |
Vasculitis is a rare but important cause of stroke, often presenting with ischemic infarcts due to vascular inflammation and stenosis. While vasculitis-related strokes more commonly occur in younger patients without traditional vascular risk factors, their presentation is highly variable, leading to complex diagnostic and management considerations. Although bi-hemispheric infarcts are often associated with hypercoagulable states or embolic sources, they can also result from vasculitis. We present a unique case of a 38-year-old male with a history of multiple autoimmune conditions who developed rapidly progressive multifocal strokes associated with cerebral vasculitis of uncertain etiology. Despite aggressive medical management, including high-dose corticosteroids, the patient experienced worsening ischemic events and further neurological decline. This case highlights the diagnostic and therapeutic challenges of vasculitis-related stroke, emphasizing the need for early recognition, comprehensive evaluation, and tailored immunosuppressive strategies to improve patient outcomes.
Keywords: Cerebral vasculitis; Ischemic stroke; Bi-hemispheric infarcts; Autoimmune disease; Atypical stroke; Neurocritical care; High-dose corticosteroids; Diagnostic challenges; Multifocal infarcts; Immunosuppressive therapy
| Introduction | ▴Top |
Vasculitis is characterized by inflammation and necrosis of blood vessels. It is classified into three general subgroups: small-vessel, medium-vessel, and large-vessel vasculitis. Stroke due to vasculitis can be either ischemic or hemorrhagic, with vasculitis-related strokes more often causing an ischemic pathology [1]. Ischemic strokes can be due to primary or secondary vasculitis which may present subacutely with systemic inflammation, focal neurological deficits, and ischemia in various vascular territories [2]. Though hemorrhagic strokes are less common, these strokes may present with a more rapid nature, with severe headaches with 10/10 intensity and/or rapid neurological decline [3, 4]. Vasculitis-related stroke tends to present in younger patients (< 50 years) with associated systemic vasculitis symptoms [5, 6]. They also may lack some of the characteristic stroke risk factors including smoking, diabetes, and hypertension, or may instead have an autoimmune presentation [6]. Compared to non-vasculitis strokes, vasculitis-related strokes imaging may show vessel abnormalities and infarcts in unusual vascular territories that may complicate determining a diagnosis and treatment plan before irreversible damage occurs.
Multiple acute stroke lesions, including bi-hemispheric infarcts, can result from multiple emboli or fragmentation of a single proximal embolic source from aortic or cardiac disease [7]. Due to their distribution across various arterial territories, bi-hemispheric infarctions strongly suggest a proximal embolic source in the heart or aorta, or an underlying systemic hypercoagulopathy. Evidence shows that even a single carotid artery lesion can cause bi-hemispheric infarctions when the stenosed artery redirects blood to the contralateral hemisphere via intracranial cross-flow through the anterior communicating artery (AcoA) [8]. Case studies have also linked bilateral hyperdense middle cerebral artery (MCA) on imaging with bi-hemispheric involvement [9]. Magnetic resonance imaging (MRI) diffusion-weighted imaging (DWI) has shown multiple acute stroke lesions in 17-30% of stroke patients, with 1.4-6.1% involving bi-hemispheric lesions in the anterior circulation [8].
Vasculitis is a rare stroke cause, yet the risk of recurrent stroke is extremely high if untreated. In fact, ischemic stroke secondary to primary inflammatory vascular conditions was only observed in six of 70 cases (9%) in a clinical series of “unusual-cause” strokes, often presenting with multifocal bi-hemispheric infarctions similar to our patient [10]. It is often overlooked in stroke causality, and varying etiologies, such as autoimmune or parainfectious vasculitis, complicate diagnosis [7]. Rapidly progressing vasculitic processes can further complicate management, potentially causing new or worsened infarcts and neurological decline. Although rare, strokes with bi-hemispheric involvement due to cerebral vasculitis add complexity to diagnosis and management. We present a rare case of rapidly progressing vasculitis causing multifocal cerebral infarcts with extensive bi-hemispheric involvement.
| Case Report | ▴Top |
A 38-year-old male with an extensive past medical history presented to the emergency department (ED) after being found unresponsive at home by his mother. His past medical history included end-stage renal disease (ESRD) on hemodialysis (HD) secondary to lupus nephritis, ulcerative colitis (UC), irritable bowel syndrome (IBS), refractory epilepsy, chronic hypertension, and a failed renal transplant in 2013. Notably, he had missed two consecutive dialysis sessions prior to admission. On arrival, he was hypertensive with systolic blood pressures ranging from 200 to 240 mm Hg. Emergency medical services (EMS) reported seizure activity during transport, requiring intubation for airway protection. Chart history shows poorly controlled epilepsy due to medication non-adherence. On initial evaluation, the patient was comatose and unresponsive to verbal comments. He was intubated for airway protection. Cranial nerve examination revealed preserved cough and gag reflexes, but absent corneal reflexes bilaterally. Pupils were sluggishly reactive and symmetric. The left upper and lower extremities withdrew to noxious stimuli, whereas no purposeful movement was appreciated on right-sided limbs. These findings were consistent with a high NIH Stroke Scale (NIHSS) score of 33, indicating severe neurological impairment. A non-contrast computed tomography (CT) scan of the head revealed evidence of prior strokes, right MCA encephalomalacia, craniotomy changes, and generalized parenchymal volume loss. Neurological examination was significant for no corneal reflexes, but preserved cough and gag reflexes with minimal withdrawal to noxious stimuli.
The patient was admitted to the medical intensive care unit (MICU) for further management and stabilization. Electroencephalography (EEG) was performed upon admission, showing burst suppression without seizure activity, and levetiracetam was initiated for seizure management. Blood pressure was controlled with a systolic target of < 160 mm Hg. Due to his unresponsiveness and continuing neurological decline, further diagnostic imaging was performed. An MRI of the brain on day 4 of admission identified a large subacute right MCA stroke and a punctate left thalamic stroke. Concurrent computed tomography angiography (CTA) of the head and neck demonstrated multi-focal vascular beading and severe stenosis of the right M2 segments, basilar artery, and bilateral posterior circulation, raising concerns for an underlying vasculitic process (Fig. 1). Given the patient’s history of UC, an autoimmune etiology was considered. Infectious vasculitis causes were also evaluated, and a lumbar puncture (LP) returned bland findings and no evidence of bacterial, viral or fungal causes. Serum varicella-zoster virus (VZV) IgG was elevated; however, cerebrospinal fluid varicella-zoster virus polymerase chain reaction (CSF VZV PCR) was negative, making active VZV vasculitis less likely. An autoimmune workup revealed a positive antinuclear antibody (ANA) titer of 1:160 but was negative for antineutrophil cytoplasmic antibody (ANCA) and other autoimmune markers.
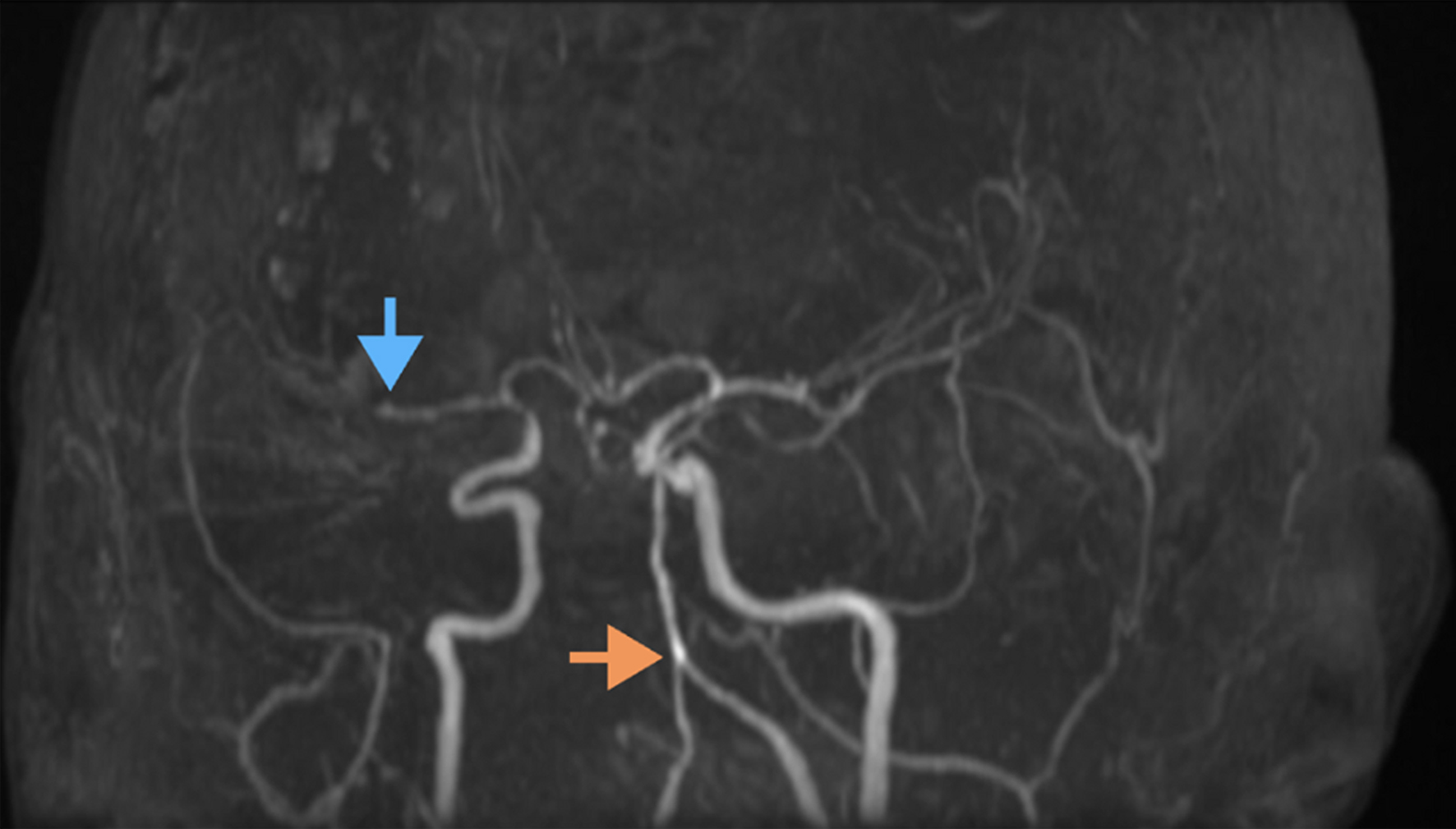 Click for large image | Figure 1. Magnetic resonance angiography (MRA) of the brain demonstrating vascular abnormalities. The orange arrow highlights tortuous and ectatic vertebral and basilar arteries, indicative of dolichoectasia. The blue arrow points to an occlusion of the right M2 segment of the middle cerebral artery. Additional findings include segmental narrowing and irregularity suggestive of underlying vasculopathy or vessel wall pathology. |
Despite medical management, the patient’s neurological status continued to deteriorate. On day 14, pupillary asymmetry and non-reactivity prompted a repeat CT scan of the head, which revealed new hypodensities in the left occipital-parietal region and the right cerebellum accompanied by increased vasogenic edema in the right parieto-occipital lobe (Fig. 2). A subsequent MRI showed progressive bilateral cytotoxic edema and laminar necrosis involving the right posterior hemisphere and bilateral basal ganglia (Fig. 3). These new findings on imaging raised suspicion of a continued rapidly progressive vasculitic process. Repeat LP results remained unremarkable and autoimmune testing was inconclusive.
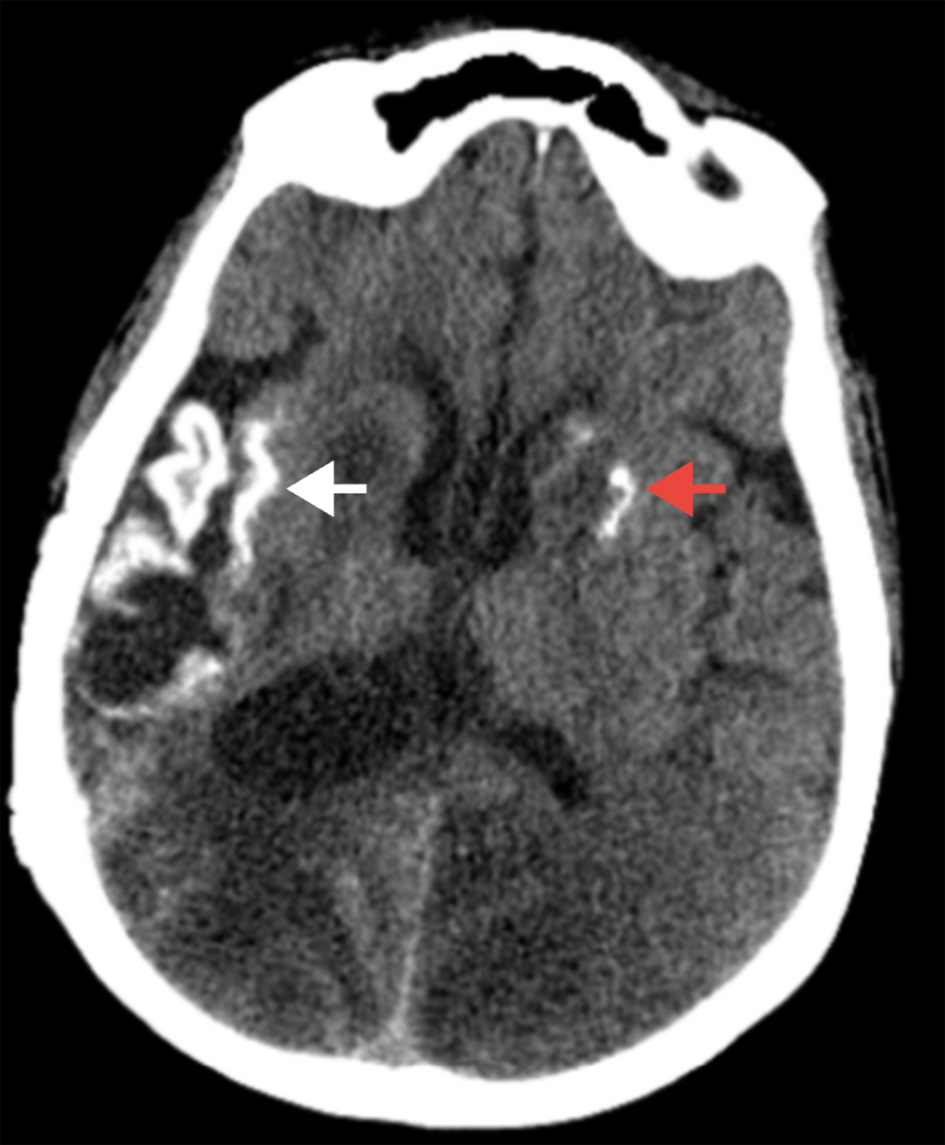 Click for large image | Figure 2. Non-contrast CT of the brain. The white arrow indicates a right-sided intracerebral hemorrhage with surrounding hypodensity consistent with cerebral edema. There is evidence of mass effect with compression of adjacent structures. There is also evidence of right posterior horn ventriculomegaly, suggestive of hydrocephalus. The red arrow demonstrates a hyperdensity in the left basal ganglia, consistent with acute hemorrhage. CT: computed tomography. |
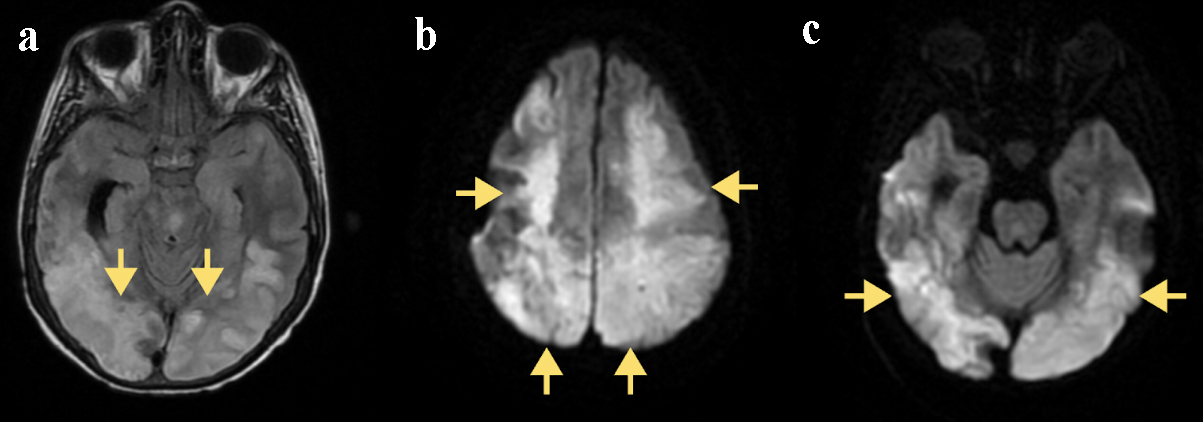 Click for large image | Figure 3. (a) Axial FLAIR MRI. Yellow arrows indicate bilateral hyperintensities in the occipital and temporal lobes, consistent with cortical and subcortical edema. (b) Diffusion-weighted MRI. Yellow arrows highlight bilateral parieto-occipital cortical and subcortical diffusion restriction. (c) Diffusion-weighted MRI. Yellow arrows identify bilateral posterior circulation infarcts with restricted diffusion in the occipital lobes. FLAIR: fluid-attenuated inversion recovery; MRI: magnetic resonance imaging. |
The patient’s hospital course was further complicated by persistent hyperkalemia and uremic encephalopathy due to previous missed HD sessions. Additional laboratory findings revealed significant leukocytosis (white blood cell (WBC) 26.6 × 109/L) with a marked left shift (band neutrophils 33.3%) and presence of nucleated red blood cells. This supported a systemic inflammatory process and possible marrow response to hypoxia. Metabolic labs were noted for elevated serum phosphorus (9.5 mg/dL) and a high anion gap (23.7), which was consistent with uremia-related acidosis. A reduced hemoglobin of 9.7 g/dL may have also reflected anemia of chronic disease in the setting of ESRD. The metabolic derangements were managed with urgent HD. Erythrocyte sedimentation rate (ESR) and platelet count were within normal limits. On day 10, the patient required further airway management and, to reduce ventilator-associated complications, underwent tracheostomy and subsequent percutaneous endoscopic gastrostomy (PEG) placement for nutritional support. Two episodes of tracheal site bleeding occurred, which were managed with packing and surgical interventions. Gastrointestinal (GI) bleeding around the PEG site was also noted, requiring epinephrine injection and cauterization during an esophagogastroduodenoscopy (EGD).
Despite aggressive therapy with high-dose corticosteroids (pulse-dose of intravenous (IV) methylprednisolone 1 g daily for 3 days, followed by oral prednisone) and symptomatic management of seizures with levetiracetam, the patient remained unresponsive. Serial imaging demonstrated progressive and extensive bi-hemispheric and brainstem involvement, consistent with ongoing strokes, worsening vasogenic edema, and laminar necrosis. Vessel imaging showed multifocal beading, severe stenoses, and diminished flow in multiple intracranial arteries. The underlying etiology of cerebral vasculitis remained uncertain due to overlapping autoimmune, parainfectious, and metabolic pathologic processes. Due to potential risk of dissection, the interventional neuroradiology team recommended no role for investigations such as angiography or tissue biopsy to help further determine a potential etiology of the vasculitis.
Goals of care discussions with the family were initiated given the poor prognosis and progression of multifocal strokes and other systemic complications. They were informed of the patient’s worsening condition which included the risk of midline shift, herniation, and cardiac arrest. After thorough conversations regarding the extent of neurological damage and poor likelihood of recovery, the patient’s family elected to transition to comfort-focused care. Do-not-resuscitate (DNR) status was implemented, and care was focused on maintaining comfort until the withdrawal of life-sustaining measures.
| Discussion | ▴Top |
Atypical presentation in vasculitis-related stroke
Our case demonstrates a rare presentation of rapidly progressing cerebral vasculitis of uncertain etiology causing multifocal strokes with bi-hemispheric and brainstem involvement. This atypical presentation highlights the diagnostic and therapeutic challenges of vasculitis-related cerebrovascular disease, especially when the underlying etiology remains undetermined. Vasculitis, a less common stroke mechanism, may present atypically compared to more common causes. Strokes from vasculitis are classified by underlying causes such as autoimmune, parainfectious, infectious, or neoplastic (Tables 1 and 2) [1, 2, 11]. A key aspect of this case is the multifocal distribution of ischemic lesions in both cerebral hemispheres, basal ganglia, and brainstem, a pattern more commonly seen in embolic strokes or hypercoagulable states [8]. Multifocal vascular beading and stenotic changes on imaging strongly suggested an underlying vasculitic process, though its exact etiology remained unclear. The spectrum of vessel beading and alternating stenosis/dilatation described by Arboix et al closely mirrors the multifocal stenoses in both anterior and posterior circulations observed on our patient’s MR angiography, reinforcing the need to maintain vasculitis high on the differential when faced with bilateral strokes of indeterminate etiology [10]. Destrebecq et al reported a case series of cryptogenic strokes attributed to primary vasculopathies, where imaging revealed acute and chronic bi-hemispheric infarcts of different ages, including thalamocapsular involvement and contrast enhancement in distal MCA and anterior cerebral artery (ACA) walls [12]. In our patient, an initial CT identified lesions in the left occipital-parietal region and right cerebellum, while subsequent MRI revealed additional involvement of the right posterior hemisphere and bilateral basal ganglia. Repeated imaging showed progressive bi-hemispheric ischemia extending to both anterior and posterior circulations and deep gray structures, suggesting an underlying systemic or central nervous system (CNS)-specific inflammatory process rather than embolic or atherosclerotic disease. Given the patient’s history of UC and positive ANA titers, an autoimmune etiology such as ANCA-associated vasculitis (AAV) was considered; however, the negative ANCA and lack of systemic vasculitic symptoms made this less likely. AAV, characterized by inflammation and necrosis of small to medium-sized vessels and often linked to myeloperoxidase (MPO)-ANCA, has been connected to ischemic stroke. Li et al reported that 10% of ischemic strokes had an ANCA-positive etiology, with a higher risk for recurrent stroke and small artery occlusion [13]. Although AAV typically involves renal and pulmonary systems, it can present as an acute ischemic stroke with bi-hemispheric involvement in younger patients lacking traditional vascular risk factors [5].
 Click to view | Table 1. Etiologies of Primary Vasculitis |
 Click to view | Table 2. Etiologies of Secondary Vasculitis |
Infectious and post-viral considerations in vasculitic stroke
VZV vasculopathy was considered given the patient’s elevated serum VZV IgG. Although uncommon, early research shows that VZV infections causing vasculitis may lead to cerebral infarctions with bi-hemispheric involvement and vasculitic stenosis [14]. The absence of CSF pleocytosis and a negative VZV DNA PCR reduced the likelihood of active infection. VZV cerebral vasculitis is typically suspected in patients with zoster or varicella who develop transient ischemic attacks or strokes due to bilateral deep vessel involvement [15]. However, lack of vesicular rash, absent CSF pleocytosis, or negative VZV DNA does not exclude the diagnosis, especially in immunosuppressed individuals [16]. Lyme neuroborreliosis, known to cause basilar artery occlusion and ischemic events in the bilateral pontine and midbrain regions, was ruled out based on negative serologic testing and absence of relevant exposure [17]. Recent studies have reported an increased incidence of multifocal ischemic strokes in patients with severe COVID-19, with bi-hemispheric strokes observed in those with severe respiratory infection and poor neurologic status due to heightened inflammation and hypercoagulability [18]. Lastly, infective endocarditis carries a high risk of multifocal or bilateral infarctions through septic embolization and mycotic aneurysm rupture but is rare according to Pujadas-Capmany cohort (two of 402 cases; 0.5%). These considerations are increasingly important as emerging evidence points to overlapping inflammatory pathways in post-infectious vasculopathy, as seen in severe COVID-19, and classical autoimmune-mediated cerebrovascular inflammation [19].
Diagnostic challenges and approach to vasculitic stroke
Diagnosing vasculitis stroke requires a multi-step approach due to its rarity and the need to exclude more common differential diagnoses and stroke mimics. This is especially true when primary stroke signs are present without systemic vasculitis symptoms, which necessitates a full workup with careful clinical evaluation [20]. Initial assessment includes a complete patient history and thorough examination to identify signs of systemic involvement, including symptoms of stroke, seizures, encephalopathy, and systemic inflammatory disorders. Diagnostic steps involve a comprehensive laboratory workup focusing on antibody-mediated diseases (e.g., ANCA, ANA titers) and inflammatory markers (e.g., complement, acute phase reactants) [21]. MRI with high-resolution vessel wall imaging (HR-VWI) is particularly useful for diagnosing large-to-medium vessel variants of primary CNS vasculitis, while small-vessel variants require brain biopsy as the gold standard for diagnosis [22, 23]. Overlapping symptoms with primary or secondary vasculitis, reversible cerebral vasoconstriction syndrome, and potential false negatives on imaging can create diagnostic pitfalls, necessitating a pragmatic treatment approach to avoid unnecessary immunosuppressive therapy in unrecognized non-inflammatory strokes (Tables 1 and 2) [3]. In our case, vessel imaging revealed multifocal beading and stenosis which suggests an inflammatory process that is not specific to any etiology. Additionally, the absence of systemic inflammatory markers such as elevated ESR and C-reactive protein (CRP) further complicated the diagnosis. The rapid deterioration and subsequent unfavorable outcome of the patient may prompt a reflection on what could have been done to achieve a more favorable prognosis. Angiography has been seen to provide useful diagnostic information distinct from MRI alone, and a complete assessment of vascular damage may warrant utilization of both modalities [24]. Isolated systemic or central nervous system vasculitis may also be differentiated by tissue sampling and biopsy. Although these procedures involve significant costs and inherent risks due to their invasive nature, tissue biopsy remains the definitive method for confirming a suspected diagnosis, particularly in cases exhibiting relentless and aggressive disease progression, as observed in our patient [25]. Although interventional neuroradiology recommended no role for further invasive investigations, a multidisciplinary discussion with a comprehensive risk-benefit assessment may have supported the necessity of further vascular imaging or biopsy, particularly considering the patient’s rapid clinical deterioration and progressive symptoms associated with vasculitis.
Acute management and therapeutic considerations
Acute management of multifocal ischemic stroke in the setting of vasculitis requires careful consideration of stroke-specific and neurocritical care management. Notably, in the Arboix et al series, vasculitis-related strokes carried no in-hospital mortality but demanded significantly longer intensive care unit stays than other unusual stroke mechanisms. However, in contrast to their cohort, our patient ultimately succumbed to her illness despite prompt and aggressive immunosuppressive and neurocritical interventions, highlighting that vasculitic strokes can be fatal and thus warrant even more urgent recognition and treatment. Unknown etiologies of stroke with a high suspicion of an autoimmune source are treated initially with IV pulse steroid therapy, followed by oral steroids alongside pulse immunosuppressive treatments [25]. Corticosteroids remain the standard first-line therapy for reducing vascular inflammation in cerebral vasculitis, typically administered as IV methylprednisolone at a dosage of 1 g daily for 3 - 5 days, followed by an oral prednisone taper at 1 mg/kg/day [26]. Aggressive corticosteroid treatment is especially indicated in rapidly progressing or severe cases. Despite a lack of direct comparative trials between 3-day and 5-day regimens, a full 5-day IV regimen is clinically preferred to achieve optimal inflammatory control [1, 27]. Insufficient initial therapy or shortened treatment courses may lead to relapse or progression of symptoms. De Boysson et al observed improved outcomes in approximately 67% of primary angiitis patients on sustained immunosuppressive therapy compared to only about 20% without prolonged therapy [27].
Aside from corticosteroids alone, combination immunosuppressive therapy has demonstrated improved outcomes in CNS vasculitis patients. Specifically, cyclophosphamide-based induction therapy followed by steroid tapering and maintenance immunosuppressants such as azathioprine has been associated with better functional outcomes compared to steroids alone [28]. Other immunomodulators such as rituximab, infliximab, and etanercept are reserved for refractory cases, demonstrating neurological improvement and fewer relapses [27]. Adjuvant therapies like IV immunoglobulin (IVIG) and plasma exchange (PLEX) have had limited evidence to support their use, but studies have indicated potential benefit for critical patients. For example, IVIG was included in the successful recovery of a patient with vasculitic brain injury due to a late diagnosis of rocky mountain spotted fever [29]. Evidence from small trials and physician consensus indicates a benefit of PLEX to be used in life-threatening systemic vasculitides; however, its utility in vasculitis-induced CNS pathologies such as stroke remains unclear.
Given the fulminant onset and rapid decline in this patient’s neurological status, it remains unclear whether an additional 2 days of IV corticosteroids or broader pharmacologic management could have improved prognosis. This uncertainty is exacerbated by limited literature evidence, complicating optimal therapeutic decision-making. Severe systemic vasculitis cases, such as ANCA-associated vasculitis, frequently require potent immunosuppressive agents like rituximab or mycophenolate mofetil to control inflammation and prevent further damage [30]. In our case, despite corticosteroid administration, neurological deterioration continued, highlighting treatment complexity due to unclear etiology. Rapid deterioration evidenced by worsening and new ischemic events on repeat imaging further complicated management. Additionally, given the increased risk of hemorrhagic transformation in vasculitis-related strokes, IV tissue plasminogen activator (tPA) administration is contraindicated due to significant intracranial hemorrhage risks [31]. Comprehensive management thus extends beyond immunomodulation, encompassing stroke-specific measures like optimized blood pressure control, neurocritical monitoring, and seizure prophylaxis, essential for mitigating additional neurological injury.
Limitations and directions for future research
Despite confirmatory imaging and clinical suspicion, the deferment of further invasive workup such as a biopsy limited diagnostic precision. The patient also presented with numerous confounding factors, such as ESRD, uremia, missed dialysis, and immunosuppressive history that may have obscured the clinical picture and masked true vasculitis processes. The anecdotal nature of single patient case reports precludes generalizability and causality. Further research should focus on developing standardized, non-invasive diagnostic criteria supported by advanced imaging techniques, and clear guidelines on when to proceed with invasive diagnostic techniques, particularly when patient condition poses significant risk.
Conclusion
This case highlights a rare and rapidly progressive presentation of vasculitis stroke with extensive bi-hemispheric involvement and a potential underlying autoimmune component, emphasizing the complexities in the diagnosis and management of cerebral vasculitis. Ischemic strokes are the more common cerebrovascular manifestation of cerebral angiitis, and this case demonstrated an aggressive, multifocal pattern of infarction that was challenging to pinpoint a single etiology. The patient’s history of UC raised suspicion for autoimmune vasculitis due to positive ANA titers, though the negative ANCA and inconclusive autoimmune markers complicated the pathogenesis of the stroke. Despite treatment with IV pulse corticosteroid therapy and supportive neurocritical care, the patient experienced a progressive neurological decline, highlighting the difficulty in managing severe vasculitis strokes, particularly in the setting of an unknown pathological source. This case reinforces the need for early recognition of vasculitis in stroke presentations, particularly in younger patients with atypical infarct patterns with a lack of systemic inflammatory features. A comprehensive diagnostic approach using the patient’s full clinical history, including advanced vessel imaging, autoimmune workup, and exclusion of infectious causes, is crucial to identify the need for timely immunosuppressive therapy. Further research into the pathophysiology and optimal treatment modalities for rapidly evolving vasculitis stroke is needed, as delayed or inadequate management may result in devastating, irreversible neurological outcomes.
Acknowledgments
None to declare.
Financial Disclosure
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Written informed consent was obtained from the patient’s legal representative for publication of this case report and accompanying images.
Authors Contributions
YA and SR were involved in concept development, study design, definition of intellectual content, literature search, clinical and experimental studies, data acquisition, data analysis, statistical analysis, manuscript preparation, editing, review, and served as guarantors. TA and CS contributed to concept development, design, definition of intellectual content, literature search, clinical and experimental studies, data acquisition, data analysis, statistical analysis, manuscript preparation, editing, review, and served as guarantors.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Berlit P. Diagnosis and treatment of cerebral vasculitis. Ther Adv Neurol Disord. 2010;3(1):29-42.
doi pubmed - Godasi R, Pang G, Chauhan S, Bollu PC. Primary central nervous system vasculitis. In: StatPearls. Treasure Island (FL) ineligible companies. 2025.
pubmed - Suthiphosuwan S, Bharatha A, Hsu CC, Lin AW, Maloney JA, Munoz DG, Palmer CA, et al. Tumefactive primary central nervous system vasculitis: imaging findings of a rare and underrecognized neuroinflammatory disease. AJNR Am J Neuroradiol. 2020;41(11):2075-2081.
doi pubmed - Liu Z, Zhou X, Zhang W, Zhou L. Resilience and its correlates among first ischemic stroke survivors at acute stage of hospitalization from a tertiary hospital in China: a cross-sectional study. Aging Ment Health. 2020;24(5):828-836.
doi pubmed - Tabakovic D, Smith R, Jayne D, Mohammad AJ. High risk of stroke in ANCA-associated vasculitis-a population-based study. Rheumatology (Oxford). 2023;62(8):2806-2812.
doi pubmed - Bukhari S, Yaghi S, Bashir Z. Stroke in young adults. J Clin Med. 2023;12(15):4999.
doi pubmed - Baird AE, Lovblad KO, Schlaug G, Edelman RR, Warach S. Multiple acute stroke syndrome: marker of embolic disease? Neurology. 2000;54(3):674-678.
doi pubmed - Saito K, Moriwaki H, Oe H, Miyashita K, Nagatsuka K, Ueno S, Naritomi H. Mechanisms of bihemispheric brain infarctions in the anterior circulation on diffusion-weighted images. AJNR Am J Neuroradiol. 2005;26(4):809-814.
pubmed - Arboix A, Bechich S, Oliveres M, Garcia-Eroles L, Massons J, Targa C. Ischemic stroke of unusual cause: clinical features, etiology and outcome. Eur J Neurol. 2001;8(2):133-139.
doi pubmed - Kuo SH, El-Hakam LM. Bilateral, hyperdense middle cerebral arteries predict bihemispheric stroke. Pediatr Neurol. 2008;39(5):361-362.
doi pubmed - Geraldes R, Santos M, Ponte C, Craven A, Barra L, Robson JC, Hammam N, et al. Stroke frequency, associated factors, and clinical features in primary systemic vasculitis: a multicentric observational study. J Neurol. 2024;271(6):3309-3320.
doi pubmed - Destrebecq V, Sadeghi N, Lubicz B, Jodaitis L, Ligot N, Naeije G. Intracranial vessel wall MRI in cryptogenic stroke and intracranial vasculitis. J Stroke Cerebrovasc Dis. 2020;29(5):104684.
doi pubmed - Li X, Cui J, Liu X, Hu Y, Wei Y, Li X, et al. Clinical characteristics of ischemic stroke in patients with positive ANCA. Neurol Asia. 2022;27(1):19-23.
- Hilt DC, Buchholz D, Krumholz A, Weiss H, Wolinsky JS. Herpes zoster ophthalmicus and delayed contralateral hemiparesis caused by cerebral angiitis: diagnosis and management approaches. Ann Neurol. 1983;14(5):543-553.
doi pubmed - Gutierrez J, Ortiz G. HIV/AIDS patients with HIV vasculopathy and VZV vasculitis: a case series. Clin Neuroradiol. 2011;21(3):145-151.
doi pubmed - Nagel MA, Bubak AN. Varicella zoster virus vasculopathy. J Infect Dis. 2018;218(suppl_2):S107-S112.
doi pubmed - Akkurt BH, Kraehling H, Nacul NG, Elsharkawy M, Schmidt-Pogoda A, Minnerup J, Stracke CP, et al. Vasculitis and ischemic stroke in lyme neuroborreliosis-interventional management approach and literature review. Brain Sci. 2023;13(10):1388.
doi pubmed - Kurian C, Mayer S, Kaur G, Sahni R, Feldstein E, Samaan M, Viswanathan D, et al. Bihemispheric ischemic strokes in patients with COVID-19. Brain Circ. 2022;8(1):10-16.
doi pubmed - Pujadas Capmany R, Arboix A, Casanas-Munoz R, Anguera-Ferrando N. Specific cardiac disorders in 402 consecutive patients with ischaemic cardioembolic stroke. Int J Cardiol. 2004;95(2-3):129-134.
doi pubmed - Berlit P, Kraemer M. Cerebral vasculitis in adults: what are the steps in order to establish the diagnosis? Red flags and pitfalls. Clin Exp Immunol. 2014;175(3):419-424.
doi pubmed - Shimoyama T, Uchino K, Calabrese LH, Hajj-Ali RA. Clinical characteristics, brain magnetic resonance imaging findings and diagnostic approach of the primary central nervous system vasculitis according to angiographic classification. Clin Exp Rheumatol. 2023;41(4):800-811.
doi pubmed - Raposo YDS, Augsten IW, Lopes MM. Diagnostic challenge in middle-aged woman with recurrent ischemic strokes: a case of primary central nervous system vasculitis and literature review. Rev Contemp. 2024;4(2):e3297.
- Uppal S, Goel S, Randhawa B, Maheshwary A. Autoimmune-associated vasculitis presenting as ischemic stroke with hemorrhagic transformation: a case report and literature review. Cureus [Internet]. 2020 [cited Mar 24, 2025]; Available from: https://www.cureus.com/articles/38036-autoimmune-associated-vasculitis-presenting-as-ischemic-stroke-with-hemorrhagic-transformation-a-case-report-and-literature-review.
- Pomper MG, Miller TJ, Stone JH, Tidmore WC, Hellmann DB. CNS vasculitis in autoimmune disease: MR imaging findings and correlation with angiography. AJNR Am J Neuroradiol. 1999;20(1):75-85.
pubmed - Parisi JE, Moore PM. The role of biopsy in vasculitis of the central nervous system. Semin Neurol. 1994;14(4):341-348.
doi pubmed - Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, Kallenberg CG, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221-232.
doi pubmed - de Boysson H, Parienti JJ, Arquizan C, Boulouis G, Gaillard N, Regent A, Neel A, et al. Maintenance therapy is associated with better long-term outcomes in adult patients with primary angiitis of the central nervous system. Rheumatology (Oxford). 2017;56(10):1684-1693.
doi pubmed - Beuker C, Strunk D, Rawal R, Schmidt-Pogoda A, Werring N, Milles L, Ruck T, et al. Primary angiitis of the CNS: a systematic review and meta-analysis. Neurol Neuroimmunol Neuroinflamm. 2021;8(6):e1093.
doi pubmed - Allen HC, Welliver RC, Sr., Fogarty MW, Gessouroun M, Henry ED. Intravenous immunoglobulin therapy for cerebral vasculitis associated with rocky mountain spotted fever. J Pediatr Intensive Care. 2017;6(2):142-144.
doi pubmed - de Boysson H, Arquizan C, Touze E, Zuber M, Boulouis G, Naggara O, Guillevin L, et al. Treatment and long-term outcomes of primary central nervous system vasculitis. Stroke. 2018;49(8):1946-1952.
doi pubmed - Esfahani NZ, Anderson DM, Pieper C, Adams HP, Jr. Intracerebral hemorrhage after IV tPA for stroke as early symptom of ANCA-associated vasculitis. eNeurologicalSci. 2017;9:1-2.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.