| Journal of Neurology Research, ISSN 1923-2845 print, 1923-2853 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Neurol Res and Elmer Press Inc |
| Journal website https://jnr.elmerpub.com |
Review
Volume 000, Number 000, October 2025, pages 000-000
Psychoeducational Therapy and Non-Pharmacological Therapeutic Intervention Approach for Pediatric Epilepsy Neuropsychiatric Comorbidities: A Neuroscience-Informed Strategy for Seizure Management
Merlion Paediatric Therapy Clinic Pte Ltd., Singapore 299552, Singapore
Manuscript submitted August 15, 2025, accepted September 10, 2025, published online October 9, 2025
Short title: Psychoeducational Therapy for Pediatric Epilepsy
doi: https://doi.org/10.14740/jnr1043
- Abstract
- Introduction
- Epilepsy Brain Mechanism and Effect of Medications
- Educational Therapy and Psychoeducation
- Common Pediatric Epilepsy Psychiatric Comorbidities and Treatment
- Practical Implementation of Pediatric Epilepsy Comorbidity Screening
- Other Non-Pharmacological Interventions (NPIs)
- Conclusions
- References
| Abstract | ▴Top |
Epilepsy is a prevalent neurological disorder frequently observed in pediatric populations. It affects approximately 0.5% to 1% of population worldwide with higher prevalence in low- and middle-income countries (LMICs). Higher incidence is also observed in children. Pediatric epilepsy is compounded by high rates of neurodevelopmental and psychiatric comorbidities, including attention-deficit/hyperactivity disorder (ADHD), depression, anxiety disorder, obsessive-compulsive disorder (OCD), autism spectrum disorder (ASD), sleeping disorders and learning disorders which negatively affect quality of life, academic achievement, and social integration. These comorbidities demonstrate a complex bidirectional relationship with epilepsy syndromes and may contribute to increased seizure propensity and clinical burden, warranting targeted therapeutic intervention alongside standard epilepsy management. Although pharmacological treatment remains a cornerstone in pediatric epilepsy management, 30% of pediatric patients with epilepsy develop refractory epilepsy (medication resistance). This article aims to synthesize current understanding of neurobiological mechanisms underlying pediatric epilepsy and associated psychiatric comorbidities. It also aims to examine the emerging role of educational and psychoeducational therapies as complementary, non-medication intervention approach to standard pharmacological treatment, and to provide a theoretical framework for integrated care approaches in pediatric drug-resistant epilepsy. Ultimately, a multimodal, family- and school-centered approach integrating pharmacological and non-pharmacological modalities is essential for optimizing long-term outcomes and quality of life in children affected by epilepsy. This narrative review was conducted through systematic literature search of PubMed, Cochrane Library, and International League Against Epilepsy (ILAE) resources from 2020 to 2025, focusing on pediatric epilepsy, psychiatric comorbidities, and non-pharmacological interventions. Search terms included “pediatric epilepsy”, “drug-resistant epilepsy”, “psychiatric comorbidities”, “psychoeducational therapy”, and “educational therapy”. This review presents theoretical frameworks based on existing literature rather than original empirical data. Claims regarding clinical efficacy require further validation through controlled studies.
Keywords: Pediatric epilepsy; Seizure; Drug resistant epilepsy; Educational therapy; Psychoeducational therapy; Psychoeducation; Psychiatric comorbidities
| Introduction | ▴Top |
Epilepsy is one of the most frequently encountered neurological disorders in pediatric populations. It is characterized by a predisposition to recurrent, unprovoked seizures caused by abnormal electrical activity in the brain as a result of aberrant neuronal excitability and network synchrony [1]. Epilepsy causes a mortality of about 125,000 each year [2]. Mortality is elevated, particularly due to sudden unexpected death in epilepsy (SUDEP) and injuries. Epilepsy affects roughly 5 - 10 per 1,000 people globally, with higher prevalence in early childhood and adolescence. Pediatric epilepsy faces a two- to threefold higher risk of premature mortality compared to peers, driven by SUDEP, status epilepticus (a seizure that persists for a sufficient length of time or is repeated frequently enough that recovery between attacks does not occur), prolong seizure activity, and accidents related to seizures [3]. About 80% of the estimated 50 million people with epilepsy (PwE) globally, including approximately 3.5 million children with epilepsy, live in low- and middle-income countries (LMICs) [4, 5].
In high-income countries, the prevalence of epilepsy is generally reported 5 - 8 per 1,000 people. In contrast, prevalence rates in LMICs are much higher, with reported median lifetime prevalence rates around 15.4 per 1,000. Some studies from Africa and Latin America reported prevalence up to 57 per 1,000 [6]. Studies have also shown that about 75% of PwE remain underreported and untreated in LMICs due to insufficient medical and healthcare support for diagnosis and medical treatment. Public health efforts targeting preventable causes, improving access to care, and addressing stigma would help reduce the global disparity in epilepsy prevalence and outcomes [1]. The higher burden in these LMICs is also attributed to increased exposure to structural etiologies (including injuries due to poor prenatal/perinatal healthcare and head trauma) and infectious etiologies (including neurocysticercosis and other central nervous system infections) [7].
The incidence of epilepsy peaks notably in the first year of life, representing half of pediatric onsets and correlating with developmental and structural brain abnormalities or severe epileptic syndromes such as West syndrome [8]. The yearly incidence rate of epilepsy in European children is approximately 70 per 100,000, though global rates vary widely from 41 to 187 per 100,000, with the highest rates occurring in less developed nations during infants’ first year [9]. The underlying causes of epilepsy vary geographically: in developing regions, birth complications and infections are primary factors, whereas in developed countries, brain tumors and head injuries are more common triggers. A secondary incidence peak emerges in adolescence, coinciding with the manifestation of idiopathic generalized epilepsies. Economic conditions play a crucial role in determining these statistics, resulting in increased epilepsy prevalence in countries with lower socioeconomic status [3].
Etiologies of epilepsy
The International League Against Epilepsy (ILAE) has identified six etiological categories for epilepsy [8, 10]. It also acknowledges that there could be multiple etiologies in PwE, reflecting the complexity of the condition.
Structural
Structural etiologies involve cortical damage and physical abnormalities in the brain creating epileptogenic foci. These cortical malformations are often focal in nature and could be identified through neuroimaging. They damage brain tissue and disrupt normal neuronal organization, causing hyperexcitability and seizures [11]. Some examples are focal cortical dysplasia or polymicrogyria, traumatic brain injury (TBI), stroke (ischemic or hemorrhagic) and brain tumors (gliomas that leads to epileptogenic foci and cause focal or generalized seizures). Hippocampal sclerosis, characterized by neuronal loss and gliosis, a hallmark of mesial temporal lobe epilepsy, is often linked to refractory seizures [12]. Perinatal brain injury, such as hypoxic-ischemic encephalopathy, as a result of oxygen and blood flow deprivation to the baby’s brain before and after birth can lead to lifelong epilepsy risk due to early cortical damage [13].
Genetic
Genetic etiologies in epilepsy refer to instances where seizures are directly caused by known or presumed genetic defects. Genes encode proteins, and a faulty gene can result in the failure to produce a protein. Genetic variations, commonly referred to as mutations, can modify a gene’s coding instructions, leading to either a dysfunctional protein or complete absence of protein production. One example would be the mutation of the sodium voltage-gated channel alpha subunit 1 (SCN1A) gene of a gamma-aminobutyric acid-ergic (GABAergic) neuron highly related to Dravet syndrome, a severe epilepsy. The SCN1A belongs to a family of genes that provide instructions for making sodium channels which transport positively charged sodium ions into neurons. It plays a key role in the neuronal cell’s ability to generate and transmit electrical signals [14]. A 2022 study reported that 80% of Dravet syndrome patients exhibit SCN1A mutations, correlating with seizure frequency [15]. Genetic associations, including KCNT1, CHD2, LGI1, CHRNA4, STXBP1, GRIN2A, DEPDC5, KCNQ3, and PRRT2, have been linked to different epilepsy syndromes that affect ion channel function, synaptic transmission, and neuronal excitability, ultimately leading to increased seizure susceptibility, as shown in Table 1 [8, 16-20].
 Click to view | Table 1. ILAE Classification of Epilepsy Types and Associated Seizure Types |
Metabolic
Metabolic conditions that affect the body’s ability to properly break down, utilize, or produce energy, vitamins, or minerals can also trigger epilepsy. These disruptions interfere with normal brain activity, impair neuronal energy production, and lead to seizures. Several of these metabolic conditions are present from birth, including glucose transporter type 1 (GLUT1, a protein that transport energy into the brain cell) deficiency, porphyria, mitochondrial diseases, and disorders that prevent the effective utilization of pyridoxine [1].
Infectious
Epilepsy is deemed to have infectious origins when it occurs due to a severe infection that spreads throughout the body’s system and causes neurological damage. Regrettably, epilepsy stemming from infections is substantially more prevalent in LMICs where vaccination programs, medications, and medical care are not readily available. Epilepsy can result from some regular infectious diseases, including tuberculosis, cerebral malaria, human immunodeficiency virus (HIV), and neurocysticercosis. The infectious diseases toxins that cause neuroinflammation and damage brain tissues can trigger seizures such as hemiconvulsion-hemiplegia-epilepsy [21].
Immune
Immune-mediated epilepsies result from autoimmune attacks on neural tissue. An autoimmune attack occurs when the body’s defense system erroneously recognizes normal, healthy parts of the body as threats and produces autoantibodies to target and attack them. The cause of the autoimmune attack is highly complex and could be attributed to genetic predisposition and infectious disease. Autoimmune encephalitis, such as anti-N-methyl-D-aspartate (NMDA) receptor or anti-leucine-rich glioma inactivated 1 (LGI1) encephalitis, involves autoantibodies targeting normal neuronal proteins, causing seizures and neuropsychiatric symptoms [22].
Unknown
The unknown etiology category applies when no structural, genetic, metabolic, infectious, or immune cause can be identified despite thorough investigation. Idiopathic generalized epilepsies, such as childhood absence epilepsy, may fall here if genetic testing is inconclusive, though many are now identified as genetic. Cryptogenic focal epilepsies involve seizures with no detectable lesion or cause on imaging or testing. This category is often temporary, as advances in diagnostics (e.g., next-generation sequencing) may reclassify cases into other etiologies [10].
Primary types of epilepsy seizures
Following the 2025 ILAE classification framework [8], epilepsy types (not individual seizure types) are categorized as focal, generalized, combined, or unknown onset. Individual seizures within these syndromes may show secondary generalization, particularly in focal epilepsies (Table 1). Focal epilepsy types are characterized by seizures originating within a localized area of one cerebral hemisphere, as shown in Figure 1. Clinical manifestations may encompass motor, sensory, autonomic, or cognitive signs, with or without impaired awareness. Structural lesions (cortical dysplasia, hippocampal sclerosis) are frequent underlying causes. Focal seizures represent approximately 60% of pediatric epilepsy cases [8, 23]. The most commonly observed focal seizures are temporal lobe epilepsy and frontal lobe epilepsy.
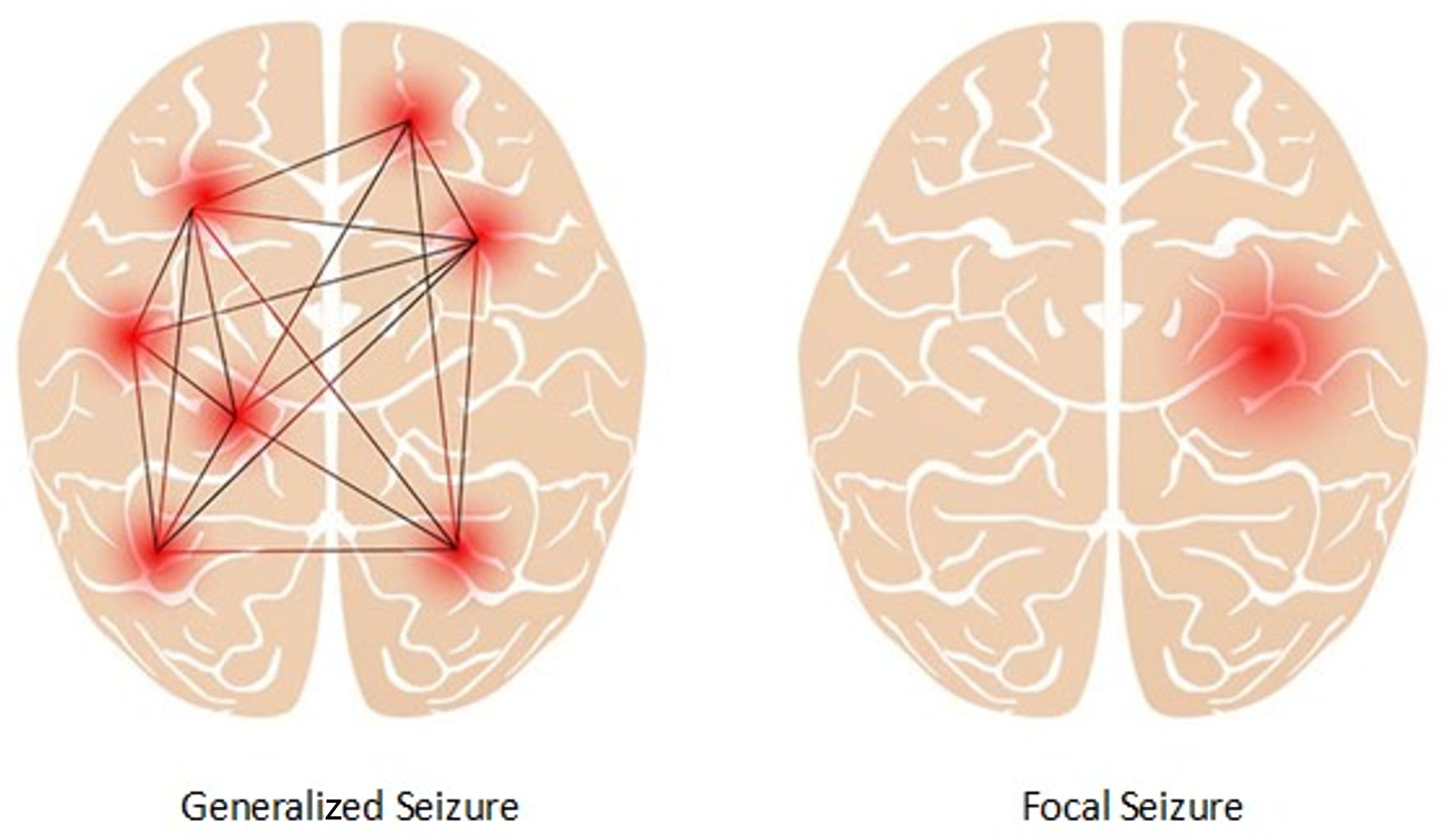 Click for large image | Figure 1. Focal seizure and generalized seizure. |
Generalized epilepsy types, on the other hand, are characterized by abnormal electrical activity that originates from both hemispheres of the brain. It involves bilateral networks from seizure onset, with seizure types including generalized tonic-clonic, absence, myoclonic, and atonic seizures. Idiopathic or genetic generalized epilepsies are prominent in this group, with typical syndromes such as childhood absence epilepsy and juvenile myoclonic epilepsy [8]. These epilepsies are strongly genetic in origin and often occur without structural brain findings.
Combined epilepsy type refers to when the seizure starts in one hemisphere of the brain but quickly spreads to involve both sides, as seen in Lennox-Gastaut syndrome. When it is unclear where the location of seizure onset is, a clinician may diagnose a patient with unknown epilepsy type; however, after more observation and testing, the diagnosis is often changed to be more specific. Lastly, to fully identify the type of epilepsy a patient is experiencing, a clinician must determine what motor symptoms the patient experiences during seizures, as well as any other non-motor symptoms. There are a wide variety of motor symptoms associated with epilepsy seizures, including tonic, atonic, and myoclonic seizures.
| Epilepsy Brain Mechanism and Effect of Medications | ▴Top |
Epilepsy seizures are primarily caused by epileptogenesis, a process that develops when there is an imbalance between brain chemicals (or neurotransmitters) that inhibit neural activity and those that promote neural excitation. At cellular level, epilepsy results from a disturbed regulation of neuronal activity disrupting the balance between excitatory glutamatergic and inhibitory GABAergic neurotransmission, as shown in Figure 2 [24]. This leads to abnormal, excessive, and synchronous firing of groups of neurons, known as neuronal hyperexcitability. Glutamate serves as the brain’s main excitatory neurotransmitter. During seizures, glutamate accumulates to dangerously high levels in the synaptic spaces between neurons, overwhelming the brain’s ability to clear it and potentially causing toxic damage. Glutamate stimulates neurons by attaching to sodium and calcium-positive ion receptors (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and NMDA) on postsynaptic neurons, causing action potential required for neuronal firing. Some antiepileptic drugs (AEDs) work by preventing glutamate from overstimulating these receptors during seizures [1, 24].
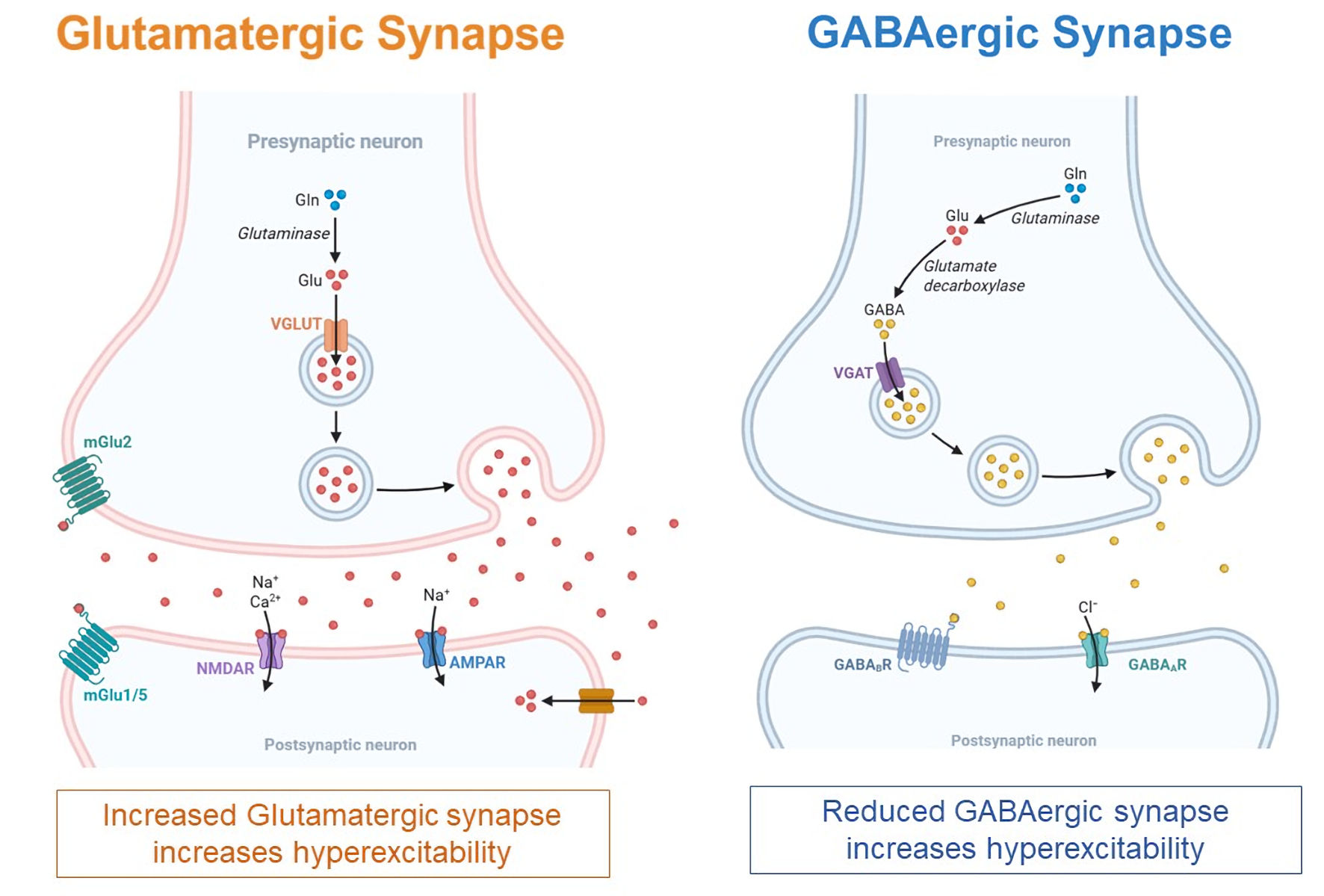 Click for large image | Figure 2. Pathophysiology of epilepsy. Decreasing inhibitory gamma-aminobutyric acid (GABA) and increasing excitatory glutamate persuade the progress and progression of epileptogenesis. |
GABA represents the brain’s primary inhibitory neurotransmitter and is synthesized from glutamate. When GABA is released from the presynaptic neuron and binds to GABA receptors on the postsynaptic neuron, it opens chloride-negative ion channels that make the postsynaptic neuron less likely to fire. PwE often have insufficient GABA levels, impaired GABA receptor function, or disrupted chloride ion balance, all of which reduce GABA’s ability to calm neural activity [1, 24]. Various AEDs target these GABA-related issues by modifying GABA production enzymes, affecting GABA breakdown processes, altering GABA transport mechanisms, or directly influencing GABA receptors.
At the network level, epileptic activity arises from aberrant synchronization of brain cortical and subcortical structures involving cortical lobes, amygdala, hippocampus, and thalamus, where maladaptive synaptic plasticity and circuit remodeling sustain seizure propagation. The dynamic interplay among localized seizure foci and larger brain networks complicates control strategies.
Function of AEDs
AEDs function by targeting specific molecules in neurons or neural circuits to regulate the abnormal brain electrical activity that triggers seizures. These drugs restore the balance between excitation and inhibition in neural pathways that becomes disrupted during epilepsy seizures [25]. They can work either by focusing on one specific target or by affecting multiple targets simultaneously. Despite the significant side effects (including ataxia, diplopia, gastrointestinal (GI) disturbance, insomnia, dizziness) associated with AEDs, they remain absolutely critical for managing severe epilepsy due to the life-threatening nature of these conditions. Seizures in epilepsy can result in status epilepticus, a life-threatening unrelenting seizure. Status epilepticus is one of the most common and serious neurological and medical emergencies in childhood associated with a high mortality rate that may lead to permanent brain damage or death. AEDs therapy is the mainstay of treatment for most patients with epilepsy, making the therapeutic benefits of immediate seizure control far outweigh the risks of side effects in these emergency situations where brain damage and death are imminent without prompt intervention [26].
Drug-resistant epilepsy (DRE)
Achieving seizure freedom is the primary therapeutic goal. According to the studies conducted by Bankole et al [27], about 70% of the PwE achieve control of epilepsy with the use of AEDs. Approximately 30% of pediatric epilepsy is refractory epilepsy or DRE, defined by failure to achieve seizure freedom despite adequate trials of two appropriate AEDs [3, 28]. Studies have also shown that related epilepsy neurodevelopmental and psychiatric comorbidities (including attention-deficit/hyperactivity disorder (ADHD), depression, anxiety disorders, obsessive-compulsive disorder (OCD), autism spectrum disorder (ASD), sleep disorders and learning disorders) and DRE are associated with increased morbidity, mortality, cognitive decline, and psychosocial impairment [29-31]. For individuals with epilepsy that is not a neurological emergency but is triggered or exacerbated by its psychiatric comorbidities, psychoeducation, educational therapy, psychoeducational therapy may serve as a valuable complementary and alternative treatment approach.
| Educational Therapy and Psychoeducation | ▴Top |
Research suggests that pediatric DRE may be associated with higher rates of mental health conditions. Psychiatric comorbidities appear to correlate with challenges in academic achievement, social integration, and quality of life, though the precise causal mechanisms require further investigation [31]. They could make it harder to control seizures effectively. Treatment for pediatric epilepsy with psychiatric comorbidities is inherently complex. Such treatment must address a constellation of interrelated challenges including cognitive executive function deficits, learning difficulties, emotional issues, social and communication problems, and behavioral disturbances. Deficits in executive function, including planning, working memory, and cognitive flexibility, compromise self-regulation and adaptive learning, making academic progress difficult. Learning difficulties further affect the child’s capacity to acquire foundational skills, leading to frustration and school avoidance. Emotional challenges, such as anxiety, depression, and mood instability, not only exacerbate cognitive and academic obstacles, but also increase the seizure propensity, severity and frequency. Social and communication issues restrict peer relationships and effective expression, heightening the risk of isolation and limiting support networks, while behavioral issues may manifest as impulsivity, inattention, aggression, or withdrawal, compounding classroom and family stress. Largely in these circumstances, psychoeducation and educational therapy becomes particularly relevant in this context.
Psychoeducation
Psychoeducation is a structured education that provides patients, families, teachers, caregivers and peers with essential knowledge and confidence about the nature, prognosis and management of the epilepsy and its associated psychiatric comorbidities, especially important for pediatric DRE cases, which require long-term, complex management beyond seizure control alone. Importantly, psychoeducation also teaches caregivers about the bidirectional impact between epilepsy and psychiatric disorders, helping them recognize early warning signs, behavioral changes, and the emotional toll on both child and family. By equipping families with knowledge and practical strategies, psychoeducation reduces stigma, fosters advocacy, and improves adherence to clinical recommendations, empowering families to make informed decisions and engage actively in multidisciplinary care. It aims to empower families, caregivers, reduce misconceptions, parental stress, stigma, and anxiety while promoting self-efficacy and adherence to treatment. Individualized psychoeducation takes into account the child’s cognitive abilities and the family’s needs, thus reinforcing social connection, reduces isolation and fosters more effective and sustainable management of epilepsy in daily life [32, 33].
Educational therapy: remediating learning and functional deficits
Educational therapy has gained official recognition from the World Health Organization (WHO), appearing under procedural code 93.82 in the WHO’s International Classification of Diseases-Ninth Edition, Clinical Modifications-Volume 3 (ICD-9, CM-3) since 1986. Unlike medical and mental health professionals who reference the ICD or the latest Diagnostic and Statistical Manual (DSM) of Mental Disorders published by the American Psychiatric Association, educational therapists work with their specialized manual: The Educator’s Diagnostic Manual (EDM) of Disabilities and Disorders. This resource uses a five-level classification system to comprehensively identify children with disabilities based on the 13 disability categories outlined in the 2004 Individuals with Disabilities Education Act (IDEA 2004) [34, 35].
Many children with pediatric epilepsy experience interruptions in schooling and academic achievement, memory loss, attentional slippage, and cognitive executive dysfunction. Frequent seizures and AEDs’ side effects may exacerbate these impairments. Educational therapists collaborate with schools and parents to implement modifications with individualized education plans (IIP), assistive technologies, and flexible instructional strategies tailored to the child’s changing neurocognitive profile that goes beyond medical treatment and behavioral therapy alone. Educational therapy specifically targets these neurocognitive and academic impairments and addresses gaps in reading, math, and writing, as well as organizational skills critical for successful learning. Through continuous assessment and dynamic adjustment, educational therapy offers practical support that directly improves academic, behavioral and psychosocial outcomes for children with DRE and complex comorbidities, while involving and helping caregivers navigate educational advocacy and resource access [36].
Psychoeducational therapy: integrating emotions regulation and behaviors management
Psychotherapy, including cognitive behavioral therapy (CBT), mindfulness-based cognitive therapy (MBCT) and dialectical behavior therapy (DBT), administered by psychologists and counselors, focuses on identifying and modifying maladaptive cognitions and behaviors through structured techniques. It is often limited, especially in pediatric DRE with comorbid neurodevelopmental and psychiatric disorders, as these disorders also require sufficient cognitive, communicative, and emotional maturity in the child with epilepsy. Psychotherapy may falter when executive dysfunction, intellectual limitations, or severe emotional and social deficits hinder abstract reasoning, sustained attention, and verbal participation. Caregivers are not directly targeted by psychotherapy as traditionally practiced. This underscores the need for integrative, multidisciplinary approaches tailored to the unique neurodevelopmental, academic, emotional, social, and behavioral profile of each pediatric epilepsy patient [36].
When emotions regulation and behaviors management (ERBM) therapy is incorporated into educational therapy, it is commonly known as psychoeducational therapy (a subcategory of educational therapy). Psychoeducational therapists who are trained in both psychotherapy and educational therapy provide individualized interventions that blend ERBM, psychotherapy techniques with educational therapy [36]. Psychoeducational therapy extends beyond information, directly addressing both psychological and educational barriers in pediatric DRE with psychiatric comorbidities. In other words, psychoeducational therapists not only work with pediatric children in addressing neurocognitive and academic deficits by devising IIP to overcome attention and memory difficulties associated with seizures and medications, they also develop coping strategies for anxiety and depression, facilitate behavioral modification, and support emotional awareness and regulation. This integrated approach helps the child with pediatric DRE build self-efficacy, skills, improve engagement, and support overall well-being. For families, joint psychoeducation sessions foster improved communication, resilience, and mutual understanding, reducing isolation and the family’s emotional burden, and facilitating a holistic, family-centered approach to disease management [37].
In summary, educational therapy appears to play an important supportive role in managing pediatric DRE with neurodevelopmental and psychiatric comorbidities by addressing the broad cognitive, learning, emotional, and social deficits these children face, thereby fostering holistic development and improving quality of life through integrative, individualized interventions. However, further evidence is necessary to confirm its essentiality.
Table 2 shows the comparison among CBT, psychoeducation, educational therapy and psychoeducational therapy, and represent synergistic, multidisciplinary frameworks that empower families to address the multifaceted challenges of pediatric DRE and psychiatric comorbidities, supporting both affected children and their caregivers in clinical, educational, and emotional domains [34-38]. Neuroscience-informed strategies are essential because they recognize that effective pediatric epilepsy treatment must address not just seizures, but the complex interactions between seizures, brain development, learning, behavior, and medication effects. This integrated approach leads to better outcomes across all domains of a child’s functioning.
 Click to view | Table 2. Comparison Among CBT, Psychoeducation, Educational Therapy and Psychoeducational Therapy |
| Common Pediatric Epilepsy Psychiatric Comorbidities and Treatment | ▴Top |
Epilepsy and its psychiatric comorbidities have a complex bidirectional relationship, in which PwE are more likely than the general population to have them, and vice versa. In the studies conducted by Medel-Matus et al [31], preliminary data evidence demonstrates that patients with DRE had “significantly higher” levels of psychiatric comorbidities compared to well-controlled epilepsy, poorly-controlled epilepsy, and control groups. These psychiatric comorbidities associated with epilepsy also increase its seizure propensity, severity and frequency through interconnected neurobiological mechanisms, which exacerbate neuronal hyperexcitability, disrupt cortical and limbic circuits, promote neuroinflammation, and impair regulatory mechanisms like GABAergic inhibition and hypothalamic-pituitary-adrenal (HPA) axis function [39]. Addressing some psychiatric comorbidities may improve epilepsy [31] and may in turn reduce SUDEP in pediatric epilepsy. The effects are compounded across ILAE etiologies, highlighting the need for comprehensive management addressing both pediatric epilepsy and its psychiatric comorbidities to improve seizure control. Table 3 shows the prevalence of the comorbidities associated with the epilepsy syndromes [8, 10, 19, 40].
 Click to view | Table 3. Effects of Comorbidities on Epileptic Seizure Severity and Frequency |
Timely detection and treatment of comorbid conditions are crucial for reducing seizure propensity, frequency and severity, as well as enhancing overall quality of life. In the normal course of the provision of therapy, educational therapists utilize tools and instruments to assess the type and severity of neurodevelopmental and learning disorders.
ADHD
Studies indicate that individuals with ADHD have a two to five times higher incidence of epilepsy compared to the general population. Seizure frequency may also be higher in those with both conditions due to overlapping triggers like stress or medication effects [40]. ADHD is characterized by dysfunction in the frontostriatal pathways, which involves the dorsolateral prefrontal cortex (dlPFC), striatum, and thalamus. These pathways, linked to dopamine and norepinephrine signaling [41], are also implicated in epileptogenic networks, particularly in frontal lobe epilepsy and self-limited epilepsy with centrotemporal spikes (SLECS) [42]. Impaired executive control, leading to poor impulse control and attention regulation, lowers the seizure threshold and increases the likelihood of both generalized (e.g., absence, tonic-clonic) and focal seizures as they amplify cortical excitability in epileptogenic zones. ADHD is associated with reduced GABAergic inhibition, which normally counteracts excitatory neural activity [20]. In epilepsy, this reduction exacerbates neuronal hyperexcitability, particularly in SLECS and frontal lobe epilepsy, leading to more frequent seizures.
Educational therapy incorporates token economies system by tailoring to the individual’s needs. Tokens are symbolic rewards (e.g., stickers, points, checkmarks, or physical items like chips) given immediately after the desired behavior is performed. These tokens can be exchanged for predetermined rewards, such as extra playtime, a small toy, screen time, or privileges (e.g., choosing a classroom activity). Rewards are individualized to be motivating, ensuring engagement and effectiveness. For example, a child with hyperactive-impulsive symptoms might earn tokens for impulse control. Token economies can reward adherence to AEDs or ADHD medications [43]. Consistent medication use stabilizes neural activity, reducing seizure frequency. By reinforcing inhibitory control, token economies improve attentional, emotional and behavioral regulation, reducing impulsive or stressful reactions that could trigger seizures. Psychoeducation could be provided to schoolteachers on medical and behavioral issues to promote supportive classroom environments and reduce stigma. They could also learn how to provide initiatives for managing seizure safety in school (e.g., seizure action plans) and enhance social inclusion and mental health [40, 44].
Depression
Depression is highly prevalent in people with temporal lobe epilepsy. Individuals with depression have a two to three times higher risk of developing epilepsy, and those with both conditions experience more frequent seizures. Depression may predispose to epilepsy, and epilepsy may exacerbate depressive symptoms due to its psychosocial burden [45]. Chronic stress from depression activates the HPA axis. HPA activation not only increases cortisol, but also reduces GABAergic inhibition and increases glutamate levels, exacerbating neuronal hyperexcitability and leading to more frequent and severe generalized seizures [46]. Brain-derived neurotrophic factor (BDNF) levels required for neurogenesis and synaptogenesis is lowered, impairing neuroplasticity and exacerbating seizure severity, especially in structural etiologies like hippocampal sclerosis [47].
Educational therapy delivers individualized strategy-focused interventions, such as metacognitive scaffolding, task-specific cognitive practice, goal setting, and exercises to strengthen inhibitory control. These targeted cognitive approaches in educational therapy support individuals in improving their planning, organization, flexible attention-shifting, and sustained mental effort for tasks. The primary objective is to enhance overall executive functioning. Educational therapists provide training on guided self-reflection by redirecting maladaptive self-referential thinking [48]. Psychoeducational therapy incorporates mindfulness-based stress reduction (MBSR), diaphragmatic breathing, and progressive muscle relaxation to manage depression related stress and emotional dysregulation [49]. These interventions help reduce HPA axis activation and cortisol levels, mitigating neuroinflammation and glutamatergic excitotoxicity that exacerbate seizures in structural (e.g., cortical malformations) and stress-sensitive epilepsy like juvenile myoclonic epilepsy [46].
Anxiety disorders
Anxiety disorders activate the amygdala and its connections to the prefrontal cortex and hippocampus, increasing glutamatergic activity and reducing GABAergic inhibition. This imbalance amplifies neuronal excitability and lowers the seizure threshold, particularly in generalized seizures and focal seizures (e.g., temporal lobe epilepsy), which directly lowers the seizure threshold [50]. The amygdala’s heightened sensitivity to stress in anxiety intensifies excitatory signaling in epileptogenic networks, increasing the likelihood of seizure initiation.
Psychoeducation involves educating individuals and families about the relationship between anxiety, stress, and seizures. Psychoeducational therapists may provide such psychoeducation and employ cognitive restructuring techniques to challenge anxiety-driven negative thoughts (e.g., catastrophic worry about seizures) and guide patients through thought-logging and perspective-taking exercises to reframe anxiety-provoking situations [51]. Visual aids, seizure diaries, and interactive discussions are engaged to enhance awareness and recognition of anxiety-related seizure triggers, most importantly, AEDs adherence [52]. These techniques aim at reducing amygdala hyperactivity and glutamatergic excitability that may trigger unnecessary seizures.
OCD
OCD is marked by intrusive, obsessive thoughts that drive specific, compulsive, and repetitive behaviors and thoughts, significantly impacting an individual’s daily life. It involves hyperactivity in the orbitofrontal cortex, anterior cingulate cortex and basal ganglia, together with serotonin and dopamine imbalances that increase neuronal excitability. Moreover, OCD can cause chronic stress and anxiety, activating the HPA axis and elevating cortisol levels, which further lower the seizure threshold by enhancing glutamatergic signaling [46].
Psychoeducation enhances patients’ awareness and control in individuals with OCD and epilepsy by teaching them to recognize and record intrusive thoughts that may trigger compulsions or seizures, allowing early identification and management of these triggers. Psychoeducational therapy can incorporate exposure and response prevention (ERP) training by gradually exposing individuals to designed triggers in a controlled environment while denying OCD patients their usual compulsive or avoidance responses. This helps desensitize the brain to these triggers and reduce anxiety and related seizure risk. ERP aims to lower the sensitivity of neural circuits over time involved in triggers and to reduce seizure frequency. By combining self-monitoring tools with ERP and cognitive strategies, educational therapy empowers patients to better regulate attention, emotions, and behaviors, ultimately contributing to improved seizure control and overall quality of life [53].
Sleep disorders
Sleep disorders disrupt the balance between excitatory and inhibitory neural activity, increasing seizure propensity. Sleep architecture, which is the cyclical pattern of non-rapid eye movement (NREM) and rapid eye movement (REM), is critical for maintaining neural stability. Sleep deprivation and disruptions, such as those caused by insomnia or sleep apnea, promote neural hyperexcitability in regions like hippocampus, a key area in epileptogenesis [54]. NREM sleep is characterized by synchronized neuronal firing, which facilitates interictal epileptiform discharges (IEDs), as abnormal electrical activities associated with seizures. This is particularly evident in epilepsies like nocturnal frontal lobe epilepsy, where seizures often occur during NREM sleep. REM sleep involves desynchronized neuronal activity and inhibits seizure propagation by reducing the likelihood of widespread neural firing. This protective effect is disrupted in sleep disorders that reduce REM sleep duration or quality. Neuroinflammation from poor sleep further amplifies neuronal hyperexcitability, particularly in immune-mediated epilepsies. Sleep disorders also disrupt AEDs metabolism, reducing efficacy and increasing seizure severity [55].
Psychoeducation plays a crucial role in mitigating these risks by raising awareness about the impact of sleep on epilepsy and equipping patients with tools to improve sleep hygiene and self-regulation. This involves creating awareness about sleep’s importance, circadian rhythms, good habits in maintaining regular sleep-wake routine, avoiding stimulating activities or screen exposure before bedtime, creating a dark and quiet sleep environment, and limiting caffeine or nicotine intake in the evening [56].
Educational therapy also utilizes sleep diaries or applications to set up personalized sleep goals (e.g., sleeping at a fixed time) and monitoring progress to improve accountability and adherence. Furthermore, psychoeducational therapy can incorporate CBT for insomnia (CBT-I) to address comorbid insomnia or anxiety [57]. This integration of self-monitoring, behavioral restructuring, and gradual exposure helps strengthen the individual’s capacity to maintain seizure control by stabilizing sleep patterns and reducing hyperexcitability linked to sleep disturbances. CBT-I stabilizes thalamocortical oscillations and melatonin signaling and in turn reducing cortical excitability that triggers focal seizures and generalized seizures, especially temporal lobe epilepsy and genetic epilepsies, where sleep deprivation significantly increases seizure frequency [58].
| Practical Implementation of Pediatric Epilepsy Comorbidity Screening | ▴Top |
Research by Duman et al [59] demonstrates that educational therapy is effective in improving both academic outcomes and psychological well-being in children with behavioral problems and mood disorders, such as attention deficit and anxiety disorders, compared to typically developing peers. Similarly, psychotherapy for anxiety disorders yields substantial improvements in global functioning according to clinician reports (d = 1.55) and moderate improvements in social functioning according to parent reports (d = 0.51) [60]. Both results indicate that early identification and intervention can meaningfully enhance daily life across multiple domains. Given these evidence-based treatment outcomes, implementing routine screening for these comorbidities in epilepsy clinics is not just beneficial but essential for optimizing long-term developmental trajectories.
The high prevalence of comorbid conditions in pediatric epilepsy necessitates systematic screening protocols, which can identify attention deficits, anxiety disorders, and learning disabilities early in the clinical pathway. Given that comorbidities are often reported at diagnosis or even before the first seizure and can progress over the course of epilepsy, healthcare teams must establish systematic comorbidities screening protocols from reactive to proactive care. Practical screening implementation should utilize validated, time-efficient instruments that can be administered in clinical settings.
Practical screening implementation should involve a multi-informant approach utilizing validated instruments administered at regular intervals throughout epilepsy care. The Piers-Harris Children’s Self-Concept Scale and Child Behavior Checklist (CBCL 6-18) have demonstrated sensitivity in detecting behavioral problems in children, while measures such as the Child Anxiety Life Interference Scale (CALIS) and Child Anxiety Impact Scale (CAIS) can effectively capture functional impairments associated with anxiety disorders [59, 60]. Screening protocols should incorporate perspectives from children, parents, and teachers to capture the full spectrum of functional difficulties, as research shows significant informant differences in reporting, with clinicians typically rating improvements more favorably than parents or children themselves. This multi-perspective approach ensures a comprehensive assessment while accounting for the varying contexts in which difficulties may manifest most prominently.
Clinical teams must establish clear referral pathways and intervention protocols following positive screens, as evidence strongly supports early therapeutic intervention for identified comorbidities. Educational therapy delivered in small group settings over 10 sessions has also shown efficacy in reducing attention deficit in children with learning disabilities, while anxiety-focused psychotherapy demonstrates significant improvements in global and social functioning across individual and group delivery formats [59, 60]. Implementation success requires training clinical staff in screening administration, establishing partnerships with educational therapists and mental health professionals, and developing systems for tracking screening results and treatment outcomes. Regular audit of screening completion rates and referral follow-through will ensure the protocol achieves its intended goal of improving comprehensive care for children with epilepsy and their families.
However, it is worth noting that resource and training constraints may present major barriers, as the screening protocols require substantial investment in staff training, validated assessment tools, and time allocation during already compressed clinic appointments.
| Other Non-Pharmacological Interventions (NPIs) | ▴Top |
NPIs effectively complement AEDs, especially for children with DRE or neurocognitive and psychosocial challenges. NPIs have gained increasing importance as they provide critical and significant benefits by enhancing symptom management, improving coping and resilience, reducing stigma, supporting academic participation, and promoting family well-being. Importantly, they address the broad neuro-psychosocial dimensions that pharmacotherapy alone cannot resolve. NPIs are essential components of comprehensive care for children with epilepsy, especially given the challenges of drug resistance, medication side effects, and the broad psychosocial and cognitive impact of these disorders. NPIs not only address symptom control directly but also empower children, families, and educators through education, behavioral modification, and mental health support. These interventions improve symptom management, promote resilience and coping, reduce stigma, and enhance overall quality of life [32].
Ketogenic diet (KD)
A high-fat, adequate-protein, low-carbohydrate diet that induces ketosis has been shown to reduce seizures in drug-resistant pediatric epilepsies, including GLUT1 deficiency, Dravet syndrome, and Lennox-Gastaut syndrome [28]. Variants such as the modified Atkins diet offer less restrictive options to improve adherence and reduce side effects [61].
Neurostimulation treatments
Neurostimulation treatments, including vagus nerve stimulation (VNS), deep brain stimulation (DBS), brain-responsive neurostimulation (bRNS) and transcranial magnetic stimulation (TMS), are established NPIs for epilepsy, especially in patients with drug-resistant forms who are not suitable candidates for resective surgery. Neurostimulation is generally well-tolerated, with mild and manageable side effects, and may also confer cognitive and mood benefits. Although these treatments rarely lead to complete seizure freedom, they substantially reduce seizure burden and improve quality of life, making them valuable adjuncts or alternatives to medication in epilepsy management. These approaches work by electrically or magnetically modulating neural circuits implicated in seizure generation [62]. VNS uses implanted devices to stimulate the vagus nerve, leading to long-term seizure frequency reduction and mood improvement [63]. DBS targets specific brain regions such as the anterior nucleus of the thalamus or hippocampus for continuous or programmed stimulation. DBS has shown consistent seizure reductions and responder rates over several years, particularly in temporal lobe and generalized epilepsies [64]. bRNS delivers responsive, targeted stimulation upon detection of seizure activity in a localized brain area. Progressive seizure reduction over time and offering periods of seizure freedom in a subset of patients have been observed in PwE [65]. Noninvasive techniques like TMS are being explored, though robust evidence for efficacy in epilepsy is limited relative to invasive modalities [66].
| Conclusions | ▴Top |
The multifaceted nature of pediatric epilepsy with its neurodevelopmental and psychiatric comorbidities demands coordinated intervention strategies that recognize the disorder’s impact on neuroconnectivity circuits and neurotransmitter systems. Neuroscience-informed strategies are essential because pediatric epilepsy is fundamentally a disorder of the developing brain, requiring approaches that consider not only seizure control but also the preservation and optimization of ongoing neural development, cognitive function, and behavioral outcomes. This comprehensive approach leads to better long-term quality of life and functional outcomes for children with epilepsy. While AEDs remain as the cornerstone of treatment, NPIs such as psychoeducation, educational therapy and psychoeducational therapy complement the cornerstone of holistic pediatric epilepsy, and its psychiatric comorbidities management may hold promise in seizure propensity reduction, by directly addressing the epilepsy comorbidities, as they target the bidirectional relationship between epilepsy and comorbidities, as well as the underlying neurobiological complexities.
However, further empirical validation is required. Current literature on psychoeducational therapy in pediatric epilepsy remains limited, with most evidence derived from studies of general population with behavioral and mood disorders, rather than the complex interplay of multiple comorbidities commonly seen in pediatric epilepsy, limiting guidance for managing patients with overlapping attention, anxiety, and learning difficulties simultaneously.
Effective intervention also depends on seamless collaboration among multidisciplinary practitioners such as neurologists, clinicians, therapists, families, educators and caregivers to create individualized treatment plans that address each child’s unique symptomatology and psychosocial context. NPIs including KD management, neurostimulation treatments (including VNS, DBS, bRNS and TMS), and mental wellness approaches complement educational and psychoeducational therapies. This comprehensive framework has the potential to reduce caregivers’ and educators’ burden and may contribute to improved developmental outcomes in children with epilepsy. However, long-term clinical studies are needed to substantiate these effects and ultimately addressing the broader public health challenges of treatment gaps and stigma through evidence-based, person-centered care.
Acknowledgments
None to declare.
Financial Disclosure
There is no funding obtained.
Conflict of Interest
There is no conflict of interest.
Author Contributions
The corresponding and sole author conceptualized, designed and wrote the paper.
Data Availability
The author declares that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Nightingale A. Epilepsy: etiology and treatment methods. The Science Journal of the Lander College of Arts and Sciences. 2025;18(2):13-27.
- Singh G, Sander JW. The global burden of epilepsy report: implications for low- and middle-income countries. Epilepsy Behav. 2020;105:106949.
doi pubmed - NICE, National Institute for Health and Care Excellence, Epilepsies in children, young people, and adults (NG217). 2025. https://www.nice.org.uk/guidance/ng217.
- WHO, World Health Organization, Epilepsy Fact Sheet. 2025. Available at: https://www.who.int/news-room/fact-sheets/detail/epilepsy.
- Vergonjeanne M, Auditeau E, Erazo D, Luna J, Gelle T, Gbessemehlan A, Boumediene F, et al. Epidemiology of epilepsy in low- and middle-income countries: experience of a standardized questionnaire over the past two decades. Neuroepidemiology. 2021;55(5):369-380.
doi pubmed - Kariuki SM, Thomas PT, Newton CR. Epilepsy stigma in children in low-income and middle-income countries. Lancet Child Adolesc Health. 2021;5(5):314-316.
doi pubmed - Sen A, Newton CR, Ngwende G. Epilepsy in low- to middle-income countries. Curr Opin Neurol. 2025;38(2):121-127.
doi pubmed - ILAE, International League Against Epilepsy, Updated Classification of Epileptic Seizures and Epilepsies. Epilepsia. 2025. https://www.ilae.org/updated-classification-epileptic-seizures-2025.
- Eslamian M, Shafiei H, Mojahed F, Bahreini A. Prevalence of epilepsy in children and adolescents worldwide: a literature overview. Health Providers. 2024;4(2):99-108.
doi - Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, Hirsch E, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512-521.
doi pubmed - Blumcke I, Budday S, Poduri A, Lal D, Kobow K, Baulac S. Neocortical development and epilepsy: insights from focal cortical dysplasia and brain tumours. Lancet Neurol. 2021;20(11):943-955.
doi pubmed - Middlebrooks EH, Gupta V, Agarwal AK, Freund BE, Messina SA, Tatum WO, Sabsevitz DS, et al. Radiologic classification of hippocampal sclerosis in epilepsy. AJNR Am J Neuroradiol. 2024;45(9):1185-1193.
doi pubmed - Herrera-Salgado JM, Reyes-Mendoza LE, Briones-Garduno JC, Gutierrez-Chavarria SA. Hypoxic-ischemic brain injury: literature review. Revista medica del Hospital General de Mexico. 2025;88(2):80-87.
doi - Veltra D, Theodorou V, Katsalouli M, Vorgia P, Niotakis G, Tsaprouni T, Pons R, et al. SCN1A channels a wide range of epileptic phenotypes: report of novel and known variants with variable presentations. Int J Mol Sci. 2024;25(11):5644.
doi pubmed - Brunklaus A, Brunger T, Feng T, Fons C, Lehikoinen A, Panagiotakaki E, Vintan MA, et al. The gain of function SCN1A disorder spectrum: novel epilepsy phenotypes and therapeutic implications. Brain. 2022;145(11):3816-3831.
doi pubmed - Wirrell EC, Specchio N, Nabbout R, Pearl PL, Riney K. Epilepsy syndromes classification. Epilepsia Open. 2025.
doi pubmed - Hirsch E, French J, Scheffer IE, Bogacz A, Alsaadi T, Sperling MR, Abdulla F, et al. ILAE definition of the Idiopathic Generalized Epilepsy Syndromes: Position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022;63(6):1475-1499.
doi pubmed - Specchio N, Wirrell EC, Scheffer IE, Nabbout R, Riney K, Samia P, Guerreiro M, et al. International League Against Epilepsy classification and definition of epilepsy syndromes with onset in childhood: Position paper by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022;63(6):1398-1442.
doi pubmed - Vinti V, Dell'Isola GB, Tascini G, Mencaroni E, Cara GD, Striano P, Verrotti A. Temporal lobe epilepsy and psychiatric comorbidity. Front Neurol. 2021;12:775781.
doi pubmed - Barone V, van Putten M, Visser GH. Absence epilepsy: Characteristics, pathophysiology, attention impairments, and the related risk of accidents. A narrative review. Epilepsy Behav. 2020;112:107342.
doi pubmed - Gong P, Karakas C, Morgan B. Child Neurology: Hemiconvulsion-Hemiplegia-Epilepsy Syndrome in the Setting of COVID-19 Infection and Multisystem Inflammatory Syndrome. Neurology. 2022;99(17):756-760.
doi pubmed - Gillinder L, Britton JW. Seizures associated with autoimmune disorders - current treatment approaches. Semin Neurol. 2025;45(2):275-286.
doi pubmed - NINDS National Institute of Neurological Disorders and Stroke, National Institute of Health. https://www.ninds.nih.gov/health-information/disorders/epilepsy-and-seizures.
- Ali NH, Al-Kuraishy HM, Al-Gareeb AI, Alnaaim SA, Alexiou A, Papadakis M, Saad HM, et al. Autophagy and autophagy signaling in Epilepsy: possible role of autophagy activator. Mol Med. 2023;29(1):142.
doi pubmed - Sanchez JD, Gomez-Carpintero J, Gonzalez JF, Menendez JC. Twenty-first century antiepileptic drugs. An overview of their targets and synthetic approaches. Eur J Med Chem. 2024;272:116476.
doi pubmed - Becker LL, Gratopp A, Prager C, Elger CE, Kaindl AM. Treatment of pediatric convulsive status epilepticus. Front Neurol. 2023;14:1175370.
doi pubmed - Bankole NDA, Dokponou YCH, De Koning R, Dalle DU, Kesici O, Egu C, Ikwuegbuenyi C, et al. Epilepsy care and outcome in low- and middle-income countries: A scoping review. J Neurosci Rural Pract. 2024;15(1):8-15.
doi pubmed - AES, American Epilepsy Society, Draft Guidelines for the Treatment of Infantile Epilepsy Syndromes. 2025. https://aesnet.org/clinical-care/clinical-guidance/guidelines.
- Mula M. Impact of psychiatric comorbidities on the treatment of epilepsies in adults. Expert Rev Neurother. 2023;23(10):895-904.
doi pubmed - Dagar A, Falcone T. Psychiatric Comorbidities in Pediatric Epilepsy. Curr Psychiatry Rep. 2020;22(12):77.
doi pubmed - Medel-Matus JS, Orozco-Suarez S, Escalante RG. Factors not considered in the study of drug-resistant epilepsy: Psychiatric comorbidities, age, and gender. Epilepsia Open. 2022;7(Suppl 1):S81-S93.
doi pubmed - Maya Kaye A. Pediatric epilepsy and psychoeducational interventions: A review of the literature. Epilepsy Behav. 2021;121(Pt A):108084.
doi pubmed - Michaelis R, Tang V, Goldstein LH, Reuber M, LaFrance WC, Jr., Lundgren T, Modi AC, et al. Psychological treatments for adults and children with epilepsy: Evidence-based recommendations by the International League Against Epilepsy Psychology Task Force. Epilepsia. 2018;59(7):1282-1302.
doi pubmed - Chua CK, Chia KH. A brief review of educational therapy & its current role: Part 1. Unlimited Human! 2023;4-5.
doi - Chua CK, Chia KH. A brief review of educational therapy & its current role: Part 2. Unlimited Human! 2023;4-5.
doi - Chia KH. A short review of psycho-educational therapy (PsyEdTx). The Asian Educational Therapist. 2024;1(2):1-8.
- Salem T, Walters KA, Verducci JS, Fristad MA. Psychoeducational and skill-building interventions for emotion dysregulation. Child Adolesc Psychiatr Clin N Am. 2021;30(3):611-622.
doi pubmed - Mubin MF, Riwanto I, Soewadi, Sakti H, Erawati E. Psychoeducational therapy with families of paranoid schizophrenia patients. Enferm Clin (Engl Ed). 2020;30(5):326-332.
doi pubmed - Rodriguez CA, Kubis MM, Arteaga CBT, Fustes OJH. Psychiatric comorbidities in epilepsy. J Epilepsy Res. 2022;12(1):21-26.
doi pubmed - He Z, Yang X, Li Y, Zhao X, Li J, Li B. Attention-deficit/hyperactivity disorder in children with epilepsy: A systematic review and meta-analysis of prevalence and risk factors. Epilepsia Open. 2024;9(4):1148-1165.
doi pubmed - Raji H, Dinesh S, Sharma S. Inside the impulsive brain: a narrative review on the role of neurobiological, hormonal and genetic factors influencing impulsivity in psychiatric disorders. Egypt J Neurol Psychiatry Neurosurg. 2025;61:4.
doi - Chen D, Liu C, Wang F, Li P, Wei Z, Nie D, Liu P, et al. Structure-function interrelationships and associated neurotransmitter profiles in drug-naive benign childhood epilepsy with central-temporal spikes patients. Eur Radiol. 2025;35(1):417-426.
doi pubmed - Cremaschi M, Avis GR, Zhao AQ, Guarnieri E, Panzeri A, Spoto A. PENguIN: A mental health application employing gamification and token economy to boost therapeutic adherence in young users. Computers in Human Behavior Reports. 2025;17:100586.
doi - Alpuche De Lille MJ, Teixeira da Silva R, Smythe T. School-based mental health and psychosocial support interventions for children and adolescents with developmental disabilities in low- and middle-income countries: A systematic review. Trop Med Int Health. 2025;30(8):763-781.
doi pubmed - Peltola J, Surges R, Voges B, von Oertzen TJ. Expert opinion on diagnosis and management of epilepsy-associated comorbidities. Epilepsia Open. 2024;9(1):15-32.
doi pubmed - Bian X, Yang W, Lin J, Jiang B, Shao X. Hypothalamic-pituitary-adrenal axis and epilepsy. J Clin Neurol. 2024;20(2):131-139.
doi pubmed - AlRuwaili R, Al-Kuraishy HM, Al-Gareeb AI, Ali NH, Alexiou A, Papadakis M, Saad HM, et al. The possible role of brain-derived neurotrophic factor in epilepsy. Neurochem Res. 2024;49(3):533-547.
doi pubmed - Tan MK, Chia KH. Brain network dysfunction and rumination in psychiatric disorders: integrative insights into educational therapy as a targeted intervention. Asian Journal of Research and Reports in Neurology. 2025;8(1):203-222.
doi - Weiss EM, Staggl S, Holzner B, Rumpold G, Dresen V, Canazei M. Preventive effect of a 7-week app-based passive psychoeducational stress management program on students. Behav Sci (Basel). 2024;14(3):180.
doi pubmed - Zeicu C, Legouhy A, Scott CA, Oliveira JFA, Winston GP, Duncan JS, Vos SB, et al. Altered amygdala volumes and microstructure in focal epilepsy patients with tonic-clonic seizures, ictal, and post-convulsive central apnea. Epilepsia. 2023;64(12):3307-3318.
doi pubmed - Buragohain D, Khichar S, Deng C, Meng Y, Chaudhary S. Analyzing metaverse-based digital therapies, their effectiveness, and potential risks in mental healthcare. Sci Rep. 2025;15(1):17066.
doi pubmed - Patel J, Feng W, Chen K, French JA, Rushton M, Hubbard S, Ren Z, et al. Use of an electronic seizure diary in a randomized, controlled trial of natalizumab in adult participants with drug-resistant focal epilepsy. Epilepsy Behav. 2021;118:107925.
doi pubmed - Himle JA, Grogan-Kaylor A, Hiller MA, Mannella KA, Norman LJ, Abelson JL, Prout A, et al. Exposure and response prevention versus stress management training for adults and adolescents with obsessive compulsive disorder: A randomized clinical trial. Behav Res Ther. 2024;172:104458.
doi pubmed - Halasz P, Szucs A. Sleep and epilepsy link by plasticity. Front Neurol. 2020;11:911.
doi pubmed - Dell'Aquila JT, Soti V. Sleep deprivation: a risk for epileptic seizures. Sleep Sci. 2022;15(2):245-249.
doi pubmed - Kacan H, Sakiz H. Impact of a psychoeducation on caregiver burden, internalized stigma, anxiety, and coping in caregivers of children with epilepsy: a randomized pilot study. Nurs Health Sci. 2025;27(2):e70095.
doi pubmed - Quintiliani MI, Imperatori C, Testani E, Losurdo A, Tamburello S, Contardi A, Della Marca G, et al. Usefulness of psychoeducational intervention in chronic insomnia: an actigraphic study. J Ment Health. 2020;29(1):20-26.
doi pubmed - Schneider CL, Hertenstein E, Nissen C. Cognitive behavioural therapy for insomnia in inpatient psychiatric care: a systematic review. J Sleep Res. 2023;32(6):e14041.
doi pubmed - Saday Duman NS, Oner O, Aysev AS. The effect of educational therapy on self-esteem and problem behaviors in children with specific learning disability. Alpha Psychiatry. 2017;18(1):85-92.
doi - Dickson SJ, Kuhnert RL, Lavell CH, Rapee RM. Impact of psychotherapy for children and adolescents with anxiety disorders on global and domain-specific functioning: a systematic review and meta-analysis. Clin Child Fam Psychol Rev. 2022;25(4):720-736.
doi pubmed - Borowicz-Reutt K, Krawczyk M, Czernia J. Ketogenic diet in the treatment of epilepsy. Nutrients. 2024;16(9):1258.
doi pubmed - Foutz TJ, Wong M. Brain stimulation treatments in epilepsy: Basic mechanisms and clinical advances. Biomed J. 2022;45(1):27-37.
doi pubmed - Austelle CW, Cox SS, Wills KE, Badran BW. Vagus nerve stimulation (VNS): recent advances and future directions. Clin Auton Res. 2024;34(6):529-547.
doi pubmed - Vetkas A, Fomenko A, Germann J, Sarica C, Iorio-Morin C, Samuel N, Yamamoto K, et al. Deep brain stimulation targets in epilepsy: Systematic review and meta-analysis of anterior and centromedian thalamic nuclei and hippocampus. Epilepsia. 2022;63(3):513-524.
doi pubmed - Zhong C, Yang K, Wang N, Yang L, Yang Z, Xu L, Wang J, et al. Advancements in Surgical Therapies for Drug-Resistant Epilepsy: A Paradigm Shift towards Precision Care. Neurol Ther. 2025;14(2):467-490.
doi pubmed - Paganin W, Contini LM. Comparison of therapeutic efficacy in depression between repetitive TMS and deep TMS. J Neural Transm (Vienna). 2025.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Neurology Research is published by Elmer Press Inc.